

4-氨基-5-氰基-6-烷胺基吡啶衍生物的合成与生物活性
收稿日期: 2015-03-18
修回日期: 2015-05-21
网络出版日期: 2015-06-12
基金资助
国家自然科学基金(Nos. 21172090, 31000867)、教育部创新团队项目部分(No. IRT0953)资助项目.
Synthesis and Biological Activity of 4-Amino-5-cyano-6-alkylamino Pyridine Derivatives
Received date: 2015-03-18
Revised date: 2015-05-21
Online published: 2015-06-12
Supported by
Project supported by the National Program on Key Basic Research Project (973 Program. No. 2010CB126100), the National Natural Science Fundation of China (No. 21172090; 31000867) and the Program for Changjiang Scholars and Innovatives Research Team in University (No. IRT0953).
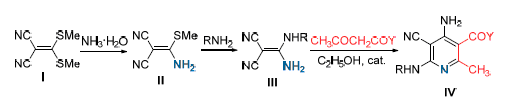
采用六水合硝酸锌作催化剂、少量的乙醇钠为助催化剂, 使用无水乙醇作为溶剂, 将2-氰基-3-烷胺基-3-氨基-丙烯腈(N,N-缩醛)与β-二羰基化合物一步关环生成了一系列目标化合物4-氨基-5-氰基-6-烷胺基吡啶衍生物. 通过1H NMR, IR, EI-MS, 元素分析等方法对所合成的化合物进行了结构表征. 代表化合物2-甲基-4-氨基-5-氰基-6-乙醇胺基-3-乙氧羰基吡啶经单晶X衍射证实了结构. 对合成的多取代吡啶衍生物化合物作了初步的农药活性测试, 测试结果表明: 所合成的多取代吡啶衍生物部分化合物表现出较好的杀菌活性, 同时也对油菜和稗草具有一定的除草活性.
刘建超 , 任青云 , 贺红武 . 4-氨基-5-氰基-6-烷胺基吡啶衍生物的合成与生物活性[J]. 有机化学, 2015 , 35(10) : 2212 -2217 . DOI: 10.6023/cjoc201503028
A series of new 4-amino-5-cyano-6-alkylamino pyridine derivatives have been designed and synthesized from 2-cyano-3-alkylamino-3-amino acrylonitriles and β-dicarbonyl compounds using Zn(NO3)2 and C2H5ONa as catalysts in anhydrous ethanol. The structures of target compounds have been confirmed by 1H NMR, EI-MS, IR spectroscopy and elemental analysis. The structure of 2-methyl-4-amino-5-cyano-6-(2-hydroxyl ethylamino)-nicotinic ethyl ester has been determined by single crystal X-ray diffraction. The results of preliminary bioassay indicated that some compounds possess potential good fungicidal activities at a dosage of 5.0×10-5 g/mL, and some compounds also possess moderate herbicidal activities against the Rape and Barnyard grass.

[1] Slawinski, J.; Szafranski, K.; Vullo, D.; Supuran, C. T. Eur. J. Med. Chem. 2013, 69, 701.
[2] Xie, Y.; Chi, H. W,; Guan, A. Y.; Liu, C. L.; Ma, H. J.; Cui, D. L. J. Agric. Food Chem. 2014, 62, 12491.
[3] Liu, Y.; Liu, Z.; Cao, X.; Liu, X.; He, H.; Yang, Y. Bioorg. Med. Chem. Lett. 2011, 21, 4779.
[4] Lee, L. F.; Sing, Y. L.; Wong, S. C. WO 9311112, 1993 [Chem. Abstr. 1993, 119, 249850].
[5] Balko, T. W.; Buysse, A. M.; Epp, J. B. WO 2003011853, 2003 [Chem. Abstr. 2003, 138, 153444].
[6] Watanabe, T.; Araki, N. WO 2005068430, 2005 [Chem. Abstr. 2005, 143 1727643].
[7] Yoshida, S.; Nagao, Y.; Watanabe, A.; Takahashi, N. Agric. Bioorg. Chem. 1980, 44, 2921.
[8] Zhong, B.; Li, Z. M.; Liu, C. L.; Zhao, W. G. Chin. J. Org. Chem. 2004, 24, 1304 (in Chinese).(钟滨, 李正名, 刘长令, 赵卫光, 有机化学, 2004, 24, 1304.)
[9] Zhong, B.; Li, Z. M.; Han, L.; Wang, S. H. Chin. J. Appl. Chem. 2005, 22, 1354 (in Chinese).(钟滨, 李正名, 韩亮, 王素华, 应用化学, 2005, 22, 1354.)
[10] Karki, R.; Thapa, P.; Kang, M. J.; Jeong, T. C.; Nam, J. M.; Kim, H.; Na, Y.; Cho, W.; Kwon, Y.; Lee, E. Bioorg. Med. Chem. 2010, 18, 3066.
[11] Onnis, V.; Cocco, M. T.; Fadda, M. T.; Congiu, C. Bioorg. Med. Chem. 2009, 17, 6158.
[12] Ando, R.; Ikegami, H.; Sakiyama, M.; Ooike, S.; Hayashi, M.; Fujino, Y.; Abe, D.; Nakamura, H.; Mishina, T.; Kata, H.; Iwase, Y.; Tomozane, H.; Morioka, M. Bioorg. Med. Chem. Lett. 2010, 20, 4709.
[13] Liu, J. C.; He, H. W.; Chen, H. L. Chin. J. Org. Chem. 2011, 31, 1208 (in Chinese).(刘建超, 贺红武, 陈荷莲, 有机化学, 2011, 31, 1208.)
[14] Ren, Q. Y.; Liang, Y. J.; He, H. W.; Fu, L. W.; Gu, Y. C. Bioorg. Med. Chem. Lett. 2009, 19, 6713.
[15] Yu, S. Y.; Li, Z. M. Chin. J. Pestic. Sci. 2001, 3, 18 (in Chinese).(余申义, 李正名, 农药学学报, 2001, 3, 18.)
[16] Song, B.; Yang, S.; Zhong, H.; Jin, L.; Hu, D.; Liu, G. J. Fluorine Chem. 2005, 126, 87.
[17] Zhao, W. G.; Liu, Z. X.; Li, Z. M.; Wang, B. L. Synth. Commun. 2003, 33, 4229.
/
| 〈 |
|
〉 |