

烯丙醇在亲核取代反应中的研究进展
收稿日期: 2015-05-05
修回日期: 2015-06-05
网络出版日期: 2015-06-19
基金资助
国家自然科学青年基金(No. 21302096 )、江苏省自然科学青年基金(No. BK20130962)、江苏高校优势学科建设工程(PAPD)资助项目.
Progress of Nucleophilic Substitution of Allylic Alcohols
Received date: 2015-05-05
Revised date: 2015-06-05
Online published: 2015-06-19
Supported by
Project supported by the Young National Natural Science Foundation of China Grant (No. 21302096), the Young Natural Science Foundation of Jiangsu Province (No. BK20130962) and the Project Fund from the Priority Academic Program Development of Jiangsu Higher, Education Institutions (PAPD).
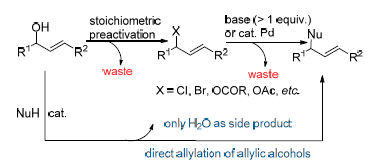
烯丙醇的亲核取代反应在有机合成化学中具有十分重要的地位, 该反应可被广泛应用于具有生物活性的药物以及天然产物的合成. 烯丙基化反应也是一类十分重要的引入C-3结构单元的合成方法学. 这种合成方法的优点在于双键的保留, 使得分子的官能团可以进行进一步转化. 传统的Tsuji-Trost烯丙基化方法会产生大量的废弃物, 相比之下直接以烯丙醇烯丙基化方法是一种绿色的合成方法. 综述了烯丙醇在路易斯酸或者布朗斯特酸催化下与不同的亲核试剂发生分子内和分子间亲核取代反应, 构筑C—X (X=C, N, O, S)键的最新研究进展, 涉及到芳基化合物、羰基化合物、氨基或者磺胺类化合物以及醇类等不同种类亲核试剂. 最后就烯丙醇烯丙基化研究及应用中存在的问题和难点对其前景进行了展望.
张小祥 , 孙小萍 , 谈继淮 , 樊辉 , 饶卫东 . 烯丙醇在亲核取代反应中的研究进展[J]. 有机化学, 2015 , 35(10) : 2049 -2058 . DOI: 10.6023/cjoc201505006
Nucleophilic substitution of allylic alcohols is very important in organic synthetic chemistry, which could be used for the synthesis of bioactive pharmaceutic compounds and natural compounds. One of the most powerful and efficient methods to introduce a C-3 unit is the allylic reaction. The advantage of this synthetic approach is the retention of the C=C bond in the product that can act as a handle for further functional group transformations. One synthetic strategy that has been often relied upon to introduce this functional group is the Tsuji-Trost reaction. However, a drawback of this method is the generation of waste products resulting from displacement of the leaving group on treating with a catalyst and/or nucleophile. Therefore, the direct allylation of allylic alcohols is considered as a green method. In this review, the latest research progress on the Lewis and Brønsted acids catalyzed intermolecular and intramolecular nucleophilic substitution for the formation of C—X (X=C, N, O, S) bond is presented, and a variety of nucleophiles, such as aromatical compounds, carbonyl compounds, amines or sulfonamides, alcohols and so on, are discussed. Finally, the problems and difficulties in research and application of allylation are discussed and then prospective is provided.

[1] Tsuji, J. Transition Metal Reagents and Catalysts, Wiley, New York, 2000.
[2] (a) Tsuji, J. Acc. Chem. Res. 1969, 2, 144.(b) Trost, B. M. Tetrahedron 1977, 33, 2615.(c) Trost, B. M. Acc. Chem. Res. 1980, 13, 385.(d) Trost, B. M.; Verhoeven, T. R. Comprehensioe Organometallic Chemistry; Pergamon: Oxford, 1982, pp. 799~938.(e) Trost, B. M. J. Organomet. Chem. 1986, 300, 263.
[3] For reviews on allylation, see: (a) Yus, M.; González-Gómez, J. C.; Foubelo, F. Chem. Rev. 2013, 113, 5595. (b) Mejuch, T.; Gilboa, N.; Gayon, E.; Wang, H.; Houk, K. N.; Marek, I. Acc. Chem. Res. 2013, 46, 1659. (c) Yus, M.; González-Gómez, J. C.; Foubelo, F. Chem. Rev. 2011, 111, 7774. (d) Tietze, L. F.; Kinzel, T.; Brazel, C. C. Acc. Chem. Res. 2009, 42, 367. For selected examples on allylation, see: (e) Tao, Z.-L.; Li, X.-H.; Han, Z.-Y.; Gong, L.-Z. J. Am. Chem. Soc. 2015, 137, 4054. (f) Deng, H.-P.; Wang, D.; Szabó, K. J. J. Org. Chem. 2015, 80, 3343.(g) Feng, T.; Si, C.; Liu, R.; Fan, X.; Wei, B. Chin. J. Org. Chem. 2013, 33, 1291 (in Chinese).(冯涛, 司长梅, 刘如成, 范翔, 魏邦国, 有机化学, 2013, 33, 1291.) (h) Min, Q.-Q.; Yin, Z.; Feng, Z.; Guo, W.-H.; Zhang, X. J. Am. Chem. Soc. 2014, 136, 1230.(i) Chen, W.; Chen, M.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 15825.(j) Sheffy, F. K.; Godschalx, J. P.; Stille, J. K. J. Am. Chem. Soc. 1984, 106, 4833. (k) Godschalx, J. P.; Stille, J. K. Tetrahedron Lett. 1980, 21, 2599. (l) Moreno-Manas, M.; Pajuelo, F.; Pleixats, R. J. Org. Chem. 1995, 60, 2396. (m) Miyaura, N.; Yamada, K.; Suginome, H.; Suzuki, A. J. Am. Chem. Soc. 1985, 107, 972. (n) Evans, P. A.; Nelson, J. D. J. Am. Chem. Soc. 1998, 120, 5581. (o) Okude, Y.; Hirano, S.; Hiyama, T.; Nozaki, H. J. Am. Chem. Soc. 1977, 99, 3179.
[4] Trost, B. M. Acc. Chem. Res. 2002, 35, 695.
[5] Bandini, M.; Tragni, M. Org. Biomol. Chem. 2009, 7, 1501.
[6] Constable, D. J. C.; Dunn, P. J.; Hayler, J. D.; Humphrey, G. R.; Leazer, J. L., Jr.; Linderman, R. J.; Lorenz, K.; Manley, J.; Pearlman, B. A.; Wells, A.; Zaks, A.; Zhang, T. Y. Green Chem. 2007, 9, 411.
[7] Tsuji, J.; Takahashi, H.; Morikawa, M. Tetrahedron Lett. 1965, 6, 4387.
[8] Tsuchimoto, T.; Tobita, K.; Hiyama,T.; Fukuzawa, S. J. Org. Chem. 1997, 62, 6997.
[9] Piao, C. R.; Zhao, Y. L.; Han, X. D.; Liu, Q. J. Org. Chem. 2008, 73, 2264.
[10] Zhang, X.; Rao, W.; Sally; Chan, P. W. H. Org. Biomol. Chem. 2009, 7, 4186.
[11] Rao, W.; Chan, P. W. H. Org. Biomol. Chem. 2008, 6, 2426.
[12] Chen, Y.; Lu, Y.; Li, G.; Liu, Y. Org. Lett. 2009, 11, 3838.
[13] McCubbin, J. A.; Hosseini, H.; Krokhin, O. V. J. Org. Chem. 2010, 75, 959.
[14] Niggemann, M.; Meel, M. J. Angew. Chem., Int. Ed. 2010, 49, 3684.
[15] Davies, H. J. Appl. Chem. 1959, 9, 137.
[16] Sanz, R.; Martínez, A.; Miguel, D.; Álvarez-Gutiérrez, J. M.; Rodríguez, F. Adv. Synth. Catal. 2006, 348, 1841.
[17] Bras, J. L.; Muzart, J. Tetrahedron 2007, 63, 7942.
[18] Liu, Y. L.; Liu, L.; Wang, Y. L.; Han, Y. C. Wang, D.; Chen, Y. J. Green Chem. 2008, 10, 635.
[19] Yasuda, M.; Somyo, T.; Baba, A. Angew. Chem., Int. Ed. 2006, 45, 793.
[20] Yadav, J. S.; Subba Reddy, B. V.; Aravind, S.; Narayana Kumar, G. G. K. S.; Reddy, A. S. Tetrahedron Lett. 2007, 48, 6117.
[21] Jana, U.; Maiti, S.; Biswas, S. Tetrahedron Lett. 2007, 48, 7160.
[22] Zhou, X.; Zhang, H.; Xie, X.; Li, Y. J. Org. Chem. 2008, 73, 3958.
[23] Guo, S.; Liu, Y. Org. Biomol. Chem. 2008, 6, 2064.
[24] Wang, J.; Zhang, L.; Jing, Y.; Huang, W.; Zhou, X. Tetrahedron Lett. 2009, 50, 4978.
[25] Namba, K.; Yamamoto, H.; Sasaki, I.; Mori, K.; Imagawa, H.; Nishizawa, M. Org. Lett. 2008, 10, 1767.
[26] Bandini, M.; Eichholzer, A.; Kotrusz, P.; Tragni, M.; Troisi, S.; Umani-Ronchi, A. Adv. Synth. Catal. 2009, 351, 319.
[27] Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9533.
[28] Yasuda, M.; Somyo, T.; Baba, A. Angew. Chem., Int. Ed. 2006, 45, 793.
[29] Rueping, M.; Nachtsheim, B. J.; Kuenkel, A. Org. Lett. 2007, 9, 825.
[30] Noji, M.; Konno, Y.; Ishii, K. J. Org. Chem. 2007, 72, 5161.
[31] Huang, W.; Wang, J.; Shen, Q.; Zhou, X. Tetrahedron Lett. 2007, 48, 3969.
[32] Jana, U.; Biswas, S.; Maiti, S. Tetrahedron Lett. 2007, 48, 4065.
[33] Rao, W.; Tay, A. H. L.; Goh, P. J.; Choy, J. M. L.; Ke, J. K.; Chan, P. W. H. Tetrahedron Lett. 2008, 49, 122.
[34] Shibuya, R.; Lin, L.; Nakahara, Y.; Mashima, K.; Ohshima, T. Angew. Chem., Int. Ed. 2014, 53, 4377.
[35] Huo, X.; Yang, G.; Liu, D.; Liu, Y.; Gridnev, I. D.; Zhang, W. Angew. Chem., Int. Ed. 2014, 53, 6776.
[36] Xie, Y.; Floreancig, P. E. Angew. Chem., Int. Ed. 2014, 53, 4926.
[37] Qin, H.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. Angew. Chem., Int. Ed. 2007, 119, 413.
[38] Guo, S.; Song, F.; Liu, Y. Synlett 2007, 964.
[39] Wu, W.; Rao, W.; Er, Y. Q.; Loh, J. K.; Poh, C.Y.; Chan, P. W. H. Tetrahedron Lett. 2008, 49, 2620.
[40] Mukherjee, P.; Widenhoefer, R. A. Org. Lett. 2010, 12, 1184.
[41] Shu, X.; Liu, X.; Xiao, H.; Ji, K.; Guo, L.; Liang, Y. Adv. Synth. Catal. 2008, 350, 243.
[42] Lu, Y.; Fu, X.; Chen, H.; Du, X.; Jia, X.; Liu, Y. Adv. Synth. Catal. 2009, 351, 129.
[43] Bauer, J. M.; Frey, W.; Peters, R. Angew. Chem., Int. Ed., 2014, 53, 7634.
[44] Zhang, X.; Teo, J. W.; Ma, D. L.; Leung, C. H.; Chan, P. W. H. Tetrahedron Lett. 2014, 55, 6703.
[45] Namba, K.; Nakagawa. Y.; Yamamoto, H.; Imagawa, H.; Nishizawa, M. Synlett 2008, 1719.
[46] Yamamoto, H.; Ho, E.; Namba, K.; Imagawa, H.; Nishizawa, M. Chem.-Eur. J. 2010, 16, 11271.
[47] Guérinot, A.; Serra-Muns, A.; Gnamm, C.; Bensoussan, C.; Reymond, S.; Cossy, J. Org. Lett. 2010, 12, 1808.
[48] Mukherjee, P.; Widenhoefer, R. A. Org. Lett. 2011, 13, 1334.
[49] Kawai, N.; Abe, R.; Matsuda, M.; Uenishi, J. J. Org. Chem. 2011, 76, 2102.
[50] Kothandaraman, P.; Foo, S. J.; Chan, P. W. H. J. Org. Chem. 2009, 74, 5947.
[51] Aponick, A.; Li, C.-Y.; Biannic, B. Org. Lett. 2008, 10, 669.
[52] Aponick, A.; Biannic, B.; Jong, M. R. Chem. Commun. 2010, 46, 6849.
[53] Bandini, M.; Monari, M.; Romaniello, A.; Tragni, M. Chem.-Eur. J. 2010, 16, 14272.
[54] Aponick, A.; Biannic, B. Org. Lett. 2011, 13, 1330.
[55] Rueping, M.; Uria, U.; Lin, M. Y.; Atodiresei, I. J. Am. Chem. Soc. 2011, 133, 3732.
[56] Satoh, T.; Ikeda, M.; Miura, M.; Nomura, M. J. Org. Chem. 1997, 62, 4877.
[57] Rao, W.; Tay, A. H. L.; Goh, P.J.; Choy, J. M. L.; Ke, J. K.; Chan, P. W. H. Tetrahedron Lett. 2008, 49, 112.
/
| 〈 |
|
〉 |