

溴化二甲基溴化硫催化下普遍、有效的芳胺甲酰化方法
收稿日期: 2015-04-12
修回日期: 2015-06-18
网络出版日期: 2015-07-02
基金资助
天津市自然科学基金(No. 12JCZDJC34300)、大学生创业训练项目(No. 201447)、天津师范大学博士基金(No. 52XB1111)资助项目.
A General and Efficient Method for the Formylation of Amines Catalyzed by Bromodimethylsulfonium Bromide
Received date: 2015-04-12
Revised date: 2015-06-18
Online published: 2015-07-02
Supported by
Project supported by the Natural Science Foundation of Tianjin City (No. 12JCZDJC34300), the College Students' Entrepreneurship Training Program (No. 201447), the Doctoral Fund of Tianjin Normal University (No. 52XB1111).
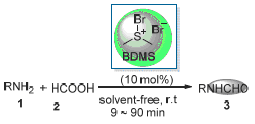
在室温、无溶剂条件下, 以溴化二甲基溴化硫(BDMS)为催化剂, 以甲酸和芳香胺类化合物为原料, 能快速、有效、便捷地实现芳香胺类化合物的甲酰化反应. 这种方法比现存的方法更环保、更高效.
关键词: 胺; N-甲酰化; 溴化二甲基溴化硫(BDMS); 无溶剂
黄海静 , 刘巨艳 , 马恩忠 , 曹映玉 . 溴化二甲基溴化硫催化下普遍、有效的芳胺甲酰化方法[J]. 有机化学, 2015 , 35(11) : 2372 -2376 . DOI: 10.6023/cjoc201504015
A fast, efficient and simple route for the N-formylation of amines has been developed by treating amines with 85% formic acid at room temperature in the presence of 5 mol% of bromodimethylsulfonium bromide as catalyst under solvent-free condition. This method provides a green and much improved protocol over the existing methods.

Key words: Amine; N-formylation; bromodimethylsulfonium bromide (BDMS); solvent-free
[1] Sheehan, J. C.; Yang, D. D. H. J. Am. Chem. Soc. 1958, 80, 1154.
[2] (a) Chen, B. C.; Bednarz, M. S.; Zhao, R.; Sundeen, J. E.; Chen, P.; Shen, Z.; Skoumbourdis, A. P.; Barrish, A. P. Tetrahedron Lett. 2000, 41, 5453.(b) Jackson, A.; Meth-Cohn, O. J. Chem. Soc., Chem. Commun. 1995, 1, 1319.
[3] (a) Waki, J.; Meienhofer, J. J. Org. Chem.1977, 42, 2019.(b) Ugi, I. Angew. Chem., Int. Ed. Engl.1982, 21, 810.
[4] Schollkopf, U. Angew. Chem., Int. Ed. Engl. 1977, 16, 339.
[5] Han, Y.; Cai, L. Tetrahedron Lett. 1997, 38, 5423.
[6] (a) Iseki, K.; Mizuno, S.; Kuroki, Y.; Kobayashi, Y. Tetrahedron 1999, 55, 977;(b) Kobayashi, S.; Nishio, K. J. Org. Chem. 1994, 59, 6620.
[7] (a) Martinez, J.; Laur, J. Synthesis1982, 979.(b) Floresheimer, A.; Kula, M. R. Monatsh. Chem.1988, 119, 1323.
[8] Fieser, L. F.; Jones, J. E. Org. Synth. Coll. 1955, III, 590.
[9] Blicke, F. F.; Lu, C.-J. J. Am. Chem. Soc. 1952, 74, 3933.
[10] Chen, F. M. F.; Benoiton, N. L. Synthesis 1979, 709.
[11] (a) Yale, H. L. J. Org. Chem. 1971, 36, 3238.(b) Kisfaludy, L.; Laszlo, O. Synthesis 1987, 510.(c) Neveux, M.; Bruneau, C.; Dixneuf, P. H. J. Chem. Soc., Perkin Trans. 1 1991, 1197.(d) Duczek, W.; Deutsch, J.; Vieth, S.; Niclas, H.-J. Synthesis 1996, 37.
[12] Schmidhammer, H.; Brossi, A. Can. J. Chem.1982, 60, 3055.
[13] Reddy, P. G.; Kumar, G. D. K.; Baskaran, S. Tetrahedron Lett.2000, 41, 9149.
[14] Strazzolini, P.; Giumanini, A. G.; Cauci, S. Tetrahedron1990, 46, 1081.
[15] Kim, J.-G.; Jang, D. O. Bull. Korean Chem. Soc. 2010, 31, 2989.
[16] Hosseni-Sarvari, M.; Sharghi, H. J. Org. Chem.2006, 71, 6652.
[17] Akbari, J.; Hekmati, M.; Sheykhan, M.; Heydari, A. ARKIVOC2009, xi, 123.
[18] Desai, B.; Danks, T. N.; Wagner, G. Tetrahedron Lett. 2005, 46, 955.
[19] Jung, S. H.; Ahn, J. H.; Park, S. K.; Choi, J. K. Bull. Korean Chem. Soc.2002, 23, 149.
[20] Das, B.; Krishnaiah, M.; Balasubramanyam, P.; Veeranjaneyulu, B.; Nandakumar, D. Tetrahedron Lett. 2008, 49, 2225.
[21] Chandra Shekhar, A.; Ravi Kumar, A.; Sathaiah, G.; Luke Paul, V.; Sridhar, M.; Shanthan Rao, P. Tetrahedron Lett.2009, 50, 7099.
[22] Rahman, M.; Kundu, D.; Hajra, A.; Majee, A. Tetrahedron Lett. 2010, 51, 2896.
[23] (a) Zhao, C. Y.; Liu, J. Y.; Wang, Y.; Zhao, X. J.; Yuan, B.; Yue, M. M. Synth. Commun. 2014, 44, 827.(b) Yue, M. M.; Liu, J. Y.; Wang, Y.; Yuan, B. Chin. J. Org. Chem. 2014, 34, 190.(c) Zhang, L. J.; Liu, J. Y.; Wang, Y.; Chin. J. Org. Chem. 2013, 33, 339.
[24] Ansari, M. I.; Hussain, M. K.; Yadav, N.; Gupta, P. K.; Hajela, K. Tetrahedron Lett. 2012, 53, 2063.
[25] Thakuria, H.; Borah, B. M.; Das, G. J. Mol. Catal. A: Chem. 2007, 274, 1.
[26] Hosseini-Sarvaia, M.; Sharghi, H. J. Org. Chem. 2006, 71, 6652.
[27] (a) Choudhury, L. H.; Parvin, T.; Khan, A. T. Tetrahedron 2009, 65, 9513.(b) Sajadia, S. M.; Mahamb, M.; Rezaeic, A. Lett. Org. Chem. 2014, 11, 49.
/
| 〈 |
|
〉 |