

万古霉素衍生物——特拉万星的合成方法改进
Process Improvement on the Synthesis of Telavancin-Derivative of Vancomycin
Received date: 2015-05-18
Revised date: 2015-06-18
Online published: 2015-07-02
刘泺 , 潘敏 , 周静珊 , 杨金纬 . 万古霉素衍生物——特拉万星的合成方法改进[J]. 有机化学, 2015 , 35(11) : 2437 -2440 . DOI: 10.6023/cjoc201505029
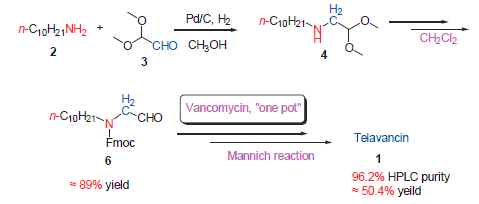
This article was the process improvement of telavancin synthesis. The key intermediate N-Fmoc-decyl-amino- acetaldehyde was obtained through continuous reaction method, followed by one-pot condensation and Mannich reaction. Telavancin hydrochloride was finnally obtained via reversed phase silica gel. The HPLC purity and total yield of this route were higher than the existing literatures. It was easy to operate, environmentally friendly, lower costs, and suitable for industrialized production.
Key words: telavancin; vancomycin; process improvement
[1] Chang, M. H.; Kish, T. D.; Fung, H. B. Clin. Ther. 2010, 32(13), 2160.
[2] Shang, X. Y.; Ruan, L. J. Chin. J. Ant. 2007, 32, 263 (in Chinese). (尚新艳, 阮丽军, 中国抗生素杂志, 2007, 32, 263.)
[3] Lee, J.; Liu, J. WO 03018607, 2003 [Chem. Abstr. 2003, 138, 205347].
[4] Liang, Y. H.; Feng, W. H. Chin. J. Synth. Chem. 2011, 19(4), 550 (in Chinese). (梁玉华, 冯文化, 合成化学, 2011, 19(4), 550.)
[5] Ma, S.; Cao, S. H. Chin. J. New Drugs 2013, 22(23), 2809 (in Chinese). (马帅, 曹胜华, 中国新药杂志, 2013, 22(23), 2809.)
[6] Michael, R. L.; Stacy, M. A.; Bettina. B. J. Ant. 2004, 57(5), 326.
[7] Liu, J; Lee, J. US 20050113561, 2005 [Chem. Abstr. 2005, 142, 451926].
[8] Leadbetter, M.; Linsell, M.; Lee, J.; Liu, J. WO 2003029270, 2003 [Chem. Abstr. 2003, 138, 287982].)
/
| 〈 |
|
〉 |