

芳基磺酰和亚磺酸类化合物参与的偶联反应研究进展
收稿日期: 2015-04-27
修回日期: 2015-06-19
网络出版日期: 2015-07-08
基金资助
国家自然科学基金(No. 21402123)、浙江省自然科学基金(Nos. LQ14B020001, LQ14B020001)、浙江省大学生科技创新活动计划(No. 2014R426012)、浙江省教育厅(No. Y201328443)和绍兴市科技计划(No. 2013B70018)资助项目.
Research Progress on Cross-Coupling with Aryl Sulfonyl andSulfinate Compounds
Received date: 2015-04-27
Revised date: 2015-06-19
Online published: 2015-07-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21402123), the Zhejiang Provincial Natural Science Foundation (Nos. LQ14B020001, LQ13B020001), the Undergraduate Science Technology Innovation of Zhejiang Province (No. 2014R426012), the Education Department of Zhejiang Province (No. Y201328443) and the Foundation of Science and Technology Bureau of Shaoxing (No. 2013B70018).
张诗浓 , 杨胜虎 , 黄乐浩 , 赵保丽 , 程凯 , 齐陈泽 . 芳基磺酰和亚磺酸类化合物参与的偶联反应研究进展[J]. 有机化学, 2015 , 35(11) : 2259 -2274 . DOI: 10.6023/cjoc201504042
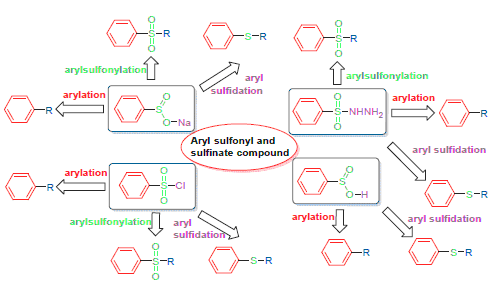
Cross-coupling reactions of aryl sulfonyl and sulfinate compounds have been well developed as an efficient synthetic method in recent years. Aryl sulfonyl chlorides, arylsulfinates and arylsulfonyl hydrazides have been used as desulfitative arylation, arylsulfonylation and reductive aryl sulfidation reagents in the synthesis of biaryls, aryl sulfones and aryl sulfides. The research progress on cross-coupling with aryl sulfonyl and sulfinate compounds combined with our work is reviewed, focusing on applications and progress in new synthetic method to construct carbon-carbon, carbon-sulfonyl and carbon-sulfur bond.
[1] Aziz, J.; Messaoudi, S.; Alami, M.; Hamze, A. Org. Biomol. Chem. 2014, 12, 9743.
[2] Vogel, P.; Dubbaka, S. R. Org. Lett. 2004, 6, 95.
[3] Vogel, P.; Dubbaka, S. R. J. Am. Chem. Soc. 2003, 125, 15292.
[4] Dubbaka, S. R.; Vogel, P. Adv. Synth. Catal. 2004, 346, 1793
[5] Vogel, P.; Dubbaka, S. R. Chem. Eur. J. 2005, 11, 2633.
[6] Vogel, P.; Dubbaka, S. R. Tetrahedron Lett. 2006, 47, 3345.
[7] Kashiwabara, T.; Tanaka, M. Tetrahedron Lett. 2005, 46, 7125.
[8] Luo, M.; Zhang, S.; Zeng, X.; Wei, Z.; Zhao, D.; Kang, T.; Zhang, W.; Yan, M. Synlett 2006, 1891.
[9] Zhang, W.; Liu, F.; Li, K.; Zhao, B. Appl. Organomet. Chem. 2014, 28, 379.
[10] Zhao, X.; Dimitrijevi?e, E.; Dong, V. M. J. Am. Chem. Soc. 2009, 131, 3466.
[11] Cheng, J.; Zhang, M.; Zhang, S.; Liu, M. Chem. Commun. 2011, 47, 11522.
[12] Yuan, K.; Doucet, H. Chem. Sci. 2014, 5, 392.
[13] Zhang, W.; Liu, F.; Zhao, B. Appl. Organomet. Chem. 2015, 29, 524.
[14] Bandgar, B. P.; Bettigeri, S. V.; Phopase, J. Org. Lett. 2004, 6, 2105.
[15] Deng, G. S.; Sun, T.; Zhou, J.; Chin. J. Org. Chem. 2012, 32, 1872 (in Chinese). (邓桂胜, 孙腾飞, 周佳, 有机化学, 2012, 32, 1872.)
[16] Zhao, X.; Dong, V. M. Angew. Chem., Int. Ed. 2011, 50, 932.
[17] Saidi, O.; Marafie, J.; Ledger, A. E. W.; Liu, P. M.; Mahon, M. F.; Kociok-Köhn, G.; Whittlesey, M. K.; Frost, C. G. J. Am. Chem. Soc. 2011, 133, 19298.
[18] Wu, A.; Song, H.; Cui, X.; Pi, C.; Du, W.; Wu, Y. Org. Lett. 2013, 15, 1270.
[19] Ge, B.; Wang, D.; Dong, W. Tetrahedron Lett. 2014, 55, 5443.
[20] Wang, D.; Ge, B.; Yu, X.; Miao, H.; Ding, Y. Chin. J. Org. Chem. 2015, 35, 676 (in Chinese). (王大伟, 葛冰洋, 余晓丽, 苗红艳, 丁玉强, 有机化学, 2015, 35, 676.)
[21] Wu, Q.; Zhao, D. B.; Qin, X. R.; Lan, J. B.; You, J. S. Chem. Commun. 2011, 47, 9188.
[22] Chen, M.; Huang, Z. T.; Zheng, Q. Y. Chem. Commun. 2012, 48, 11686.
[23] Deng, G. J.; Zhou, X.; Luo, J.; Liu, J.; Peng, S. Org. Lett. 2011. 13, 1432.
[24] Liu, S.; Bai, Y.; Cao, X.; Xiao, F.; Deng, G. J. Chem. Commun. 2013, 49, 7501.
[25] Xu, Y.; Zhao, J.; Tang, X.; Wu, W.; Jiang, H. Adv. Synth. Catal. 2014, 356, 2029.
[26] Forgione, P.; Ortgies, D. H.; Barthelme, A.; Aly, S.; Desharnais, B.; Rioux, S. Synthesis 2013, 45, 694.
[27] Cheng, K.; Hu, S.; Zhao, B.; Zhang, X.-M. Qi, C. J. Org. Chem. 2013, 78, 5022.
[28] Cheng, K.; Yu, H.-Z.; Zhao, B.; Hu, S.; Zhang, X.-M.; Qi, C. RSC Adv. 2014, 4, 57923.
[29] Luo, M.; Rao, B.; Zhang, W.; Hu, L. Green Chem. 2012, 14, 3436.
[30] Li, C. J.; Rao, H.; Yang, L.; Shuai, Q. Adv. Synth. Catal. 2011, 353, 1701.
[31] Deng, G. J.; Li. C. J.; Liu, J.; Zhou, X.; Rao, H.; Xiao, F. Chem. Eur. J. 2011, 17, 7996.
[32] Behrends, M.; Sävmarker, J.; Sjöberg, P. J. R.; Larhed, M. ACS Catal. 2011, 1, 1455.
[33] You, J.; Liu, B.; Guo, Q.; Cheng, Y.; Lan, J. Chem. Eur. J. 2011, 17, 13415.
[34] Deng, G. J.; Chen, R.; Liu, S.; Liu, X.; Luo, Y. Org. Biomol. Chem. 2011, 9, 7675.
[35] Wang, L.; Wang, M.; Li, D.; Zhou, W. Tetrahedron 2012, 68, 1926.
[36] Deng, G. J.; Luo, H. A.; Wu, M.; Luo, J.; Xiao, F.; Zhang, S. Adv. Synth. Catal. 2012, 354, 335.
[37] Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Parisi, L. M. Synlett 2003, 361.
[38] Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Parisi, L. M.; Bernini, R. J. Org. Chem. 2004, 69, 5608.
[39] Suzuki, H.; Abe, H. Tetrahedron Lett. 1995, 36, 6239.
[40] Wang, Z.; Baskin, J. M. Org. Lett. 2002, 4, 4423.
[41] Ma, D.; Zhu, W. J. Org. Chem. 2005, 70, 2696.
[42] Guo, S.; Yuan, Y. Synlett 2011, 2750.
[43] Beaulieu, C.; Guay, D.; Wang, Z.; Evan, D. A. Tetrahedron Lett. 2004, 45, 3233.
[44] Batey, R. A.; Huang, F. Tetrahedron 2007, 63, 7667.
[45] Tse, M. K.; Kar, A.; Sayyed, I. A.; Lo, W. F.; Kaiser, H. M.; Beller, M. Org. Lett. 2007, 9, 3405.
[46] Kantam, M. L.; Neelima, B.; Sreedhar, B.; Chakravarti, R. Synlett 2008, 1455.
[47] Yang, H.; Li, Y.; Jiang, M.; Wang, J.; Fu, H. Chem. Eur. J. 2011, 17, 5652.
[48] Zhang, W.; Li, K.; Zhao, B. J. Chem. Res. 2014, 38, 269.
[49] Kuwano, R.; Kondo, Y.; Shirahama, T. Org. Lett. 2005, 7, 2973.
[50] Sreedhar, B.; Reddy, M. A.; Reddy, P. S. Adv. Synth. Catal. 2010, 352, 1861.
[51] Umierski, N.; Manolikakes, G. Org. Lett. 2013, 15, 188.
[52] Xu, Y.; Zhao, J.; Tang, X.; Wu, W.; Jiang, H. Adv. Synth. Catal. 2014, 356, 2029.
[53] Xiao, F. H.; Xie, H.; Liu, S. W.; Deng, G. J. Adv. Synth. Catal. 2014, 356, 364.
[54] Yang, F. L.; Ma, X. T.; Tian, S. K. Chem. Eur. J. 2012, 18, 1582.
[55] Chen, W.; Chen, H.; Xiao, F.; Deng, G. J. Org. Biomol. Chem. 2013, 11, 4295.
[56] You, J.; Liu, B.; Li, J.; Song, F. Chem. Eur. J. 2012, 18, 10830.
[57] Yu, X.; Li, X.; Wan, B. Org. Biomol. Chem. 2012, 10, 7479.
[58] Kwong, F. Y.; Yuen, O. Y.; So, C. M.; Wong, W. T. Synlett 2012, 2714.
[59] Wu, X.-M.; Wang, Y. Synlett 2014, 25, 1163.
[60] Li, X.; Xu, Y.; Wu, W.; Jiang, C.; Qi, C.; Jiang, H. Chem. Eur. J. 2014, 20, 7911.
[61] Tang, S.; Wu, Y.; Liao, W.; Bai, R.; Liu, C.; Lei, A. Chem. Commun. 2014, 50, 4496.
[62] Wang, H.; Wei, W.; Liu, C.; Yang, D.; Wen, J.; You, J.; Suo, Y. Chem. Commun. 2013, 49, 10239.
[63] Yang, Y.; Tang, L.; Zhang, S.; Guo, X.; Zha, Z.; Wang, Z. Green Chem. 2014, 16, 4106.
[64] Li, X.; Shi, X.; Fang, M.; Xu, X. J. Org. Chem. 2013, 78, 9499.
[65] Wang, J.; Xu, C.; Wei, S.; Zhao, B.; Cheng, K. Chin. J. Org. Chem. 2014, 34, 767 (in Chinese).(王建伟, 徐程见, 韦珊红, 赵保丽, 程凯, 有机化学, 2014, 34, 767.)
[66] Singh, N.; Singh, R.; Raghuvanshi, D. S.; Singh, K. N. Org. Lett. 2013, 15, 5874.
[67] Singh, R.; Allam, B. K.; Singh, N.; Kumari, K.; Singh, S. K.; Singh, K. N. Adv. Synth. Catal. 2015, 357, 1181.
[68] Singh, R.; Raghuvanshi, D. S.; Singh, K. N. Org. Lett. 2013, 15, 4202.
[69] Yang, F. L.; Wang, F. X.; Wang, T. T.; Wang, Y. J.; Tian, S. K. Chem. Commun. 2014, 50, 2111.
[70] Guo, S.; He, W.; Xiang, J.; Yuan, Y. Chem. Commun. 2014, 50, 8578.
[71] Zhao, X.; Zhang, L.; Li, T.; Liu, G.; Wang, H.; Lu, K. Chem. Commun. 2014, 50, 13121.
[72] Yan, R.; Kang, X.; Yan, R.; Yu, G.; Pang, X.; Liu, X.; Li, X.; Xiang, L.; Huang. G. J. Org. Chem. 2014, 79, 10605.
[73] Tian, S. K.; Yang, F. L. Angew. Chem., Int. Ed. 2013, 52, 4929.
[74] Wang, G. W.; Miao, T. Chem. Eur. J. 2011, 17, 5787.
[75] Wang, G. W.; Miao, T. Chem. Commun., 2011, 47, 9501.
[76] Duan, C.; Li, Y.; Wang, H.; Zhang, R.; Jin, K.; Zhao, D. J. Org. Chem. 2012, 77, 4849.
[77] Liu, C.-R.; Ding, L.-H. Org. Biomol. Chem. 2015, 13, 2251.
/
| 〈 |
|
〉 |