

AgOTf催化的一锅法合成1-(1H-吡咯-3-基)-1,2-二氢异喹啉衍生物
收稿日期: 2015-05-23
修回日期: 2015-06-25
网络出版日期: 2015-07-08
基金资助
南通市自然科学基金(No. BK2014069)、江苏省高校优势学科资(PAPD)资助项目.
Synthesis of 1-(1H-Pyrrol-3-yl)-1,2-dihydroisoquinoline Derivatives via AgOTf-Catalyzed One-Pot Reaction
Received date: 2015-05-23
Revised date: 2015-06-25
Online published: 2015-07-08
Supported by
Project supported by the Natural Science Foundation of Nantong City (No. BK2014069) and the Priority Academic Program Development of Jiangsu Higher Education Instititutions (PAPD).
马金龙 , 翟艳光 , 朱俐 , 赵育 . AgOTf催化的一锅法合成1-(1H-吡咯-3-基)-1,2-二氢异喹啉衍生物[J]. 有机化学, 2015 , 35(11) : 2366 -2371 . DOI: 10.6023/cjoc201505034
Three-component reactions of 2-alkynylbenzaldehydes, amines and pyrrole catalyzed by AgOTf give 1-(1H-pyr- rol-3-yl)-1,2-dihydroisoquinolines in good yields. The chemical structures of these compounds were confirmed by 1H NMR, 13C NMR and HRMS techniques
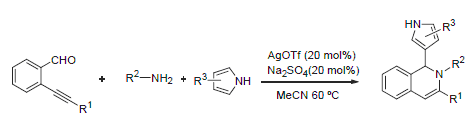
Key words: 2-alkynylbenzaldehyde; amine; pyrrole; 1,2-dihydroisoquinoline; synthesize
[1] Cao, S.; Jing, Y. F.; Liu, Y. Y.; Wan, J. P. Chin. J. Org. Chem. 2014, 34, 876 (in Chinese).(曹硕, 景艳锋, 刘云云, 万结平, 有机化学, 2014, 34, 876.)
[2] Tang, M.; Xing, D.; Cai, M. Q.; Hu, W. H. Chin. J. Org. Chem. 2014, 34, 1268 (in Chinese).(唐敏, 邢栋, 蔡茂强, 胡文浩, 有机化学, 2014, 34, 1268.)
[3] Li, X. W.; Wang, C. Y.; Zheng, L. Y. Chin. J. Org. Chem.2006, 26, 1144 (in Chinese).(李雄武, 汪朝阳, 郑绿茵, 有机化学, 2006, 26, 1144.)
[4] Domling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168.
[5] Ramesh, P.; Reddy, N. S.; Venkateswarlu, Y. J. Nat. Prod. 1999, 62, 780.
[6] Ding, Q.; Wu, J. Org. Lett. 2007, 9, 4959.
[7] Gao, K.; Wu, J. J. Org. Chem. 2007, 72, 8611.
[8] Nataliya, A. M.; Raffaella, M.; Benjamin, N.; Gerald, H. L.; Richard, C. L. Comb. Sci. 2011, 13, 265.
[9] Asao, N.; Yudha, S. S.; Nogami, T.; Yamamoto, Y. Angew. Chem., Int. Ed. 2005, 44, 5526.
[10] Yu, X.X.; Wu, J. J. Comb. Chem. 2010, 12, 238.
[11] Enders, D.; Narine, A.; Toulgoat, F.; Bisschops, T. Angew. Chem., Int. Ed. 2008, 47, 5661.
[12] Lin, H.; Sun, X.-W. Tetrahedron Lett. 2008, 49, 5343.
[13] Alonso, I.; Esquivias, J.; Gomez-Arrayas, R.; Carretero, J. C. J. Org. Chem. 2008, 73, 6400.
[14] Martinez, R.; Espinosa, A.; Tarraga, A.; Molina, P. Tetrahedron2008, 64, 2184.
[15] Podder, S.; Choudhury, J.; Roy, U. K.; Roy, S. J. Org. Chem. 2007, 72, 3100.
[16] Hagiwara, H.; Sekifuji, M.; Hoshi, T.; Qiao, K.; Yokoyama, C. Synlett 2007, 1320.
[17] Nair, V.; Vidya, N.; Abhilash, K. G. Synthesis 2006, 3647.
[18] Biaggi, C.; Benaglia, M.; Raimondi, L.; Cozzi, F. Tetrahedron 2006, 62, 12375.
[19] Shirakawa, S.; Kobayashi, S. Org. Lett. 2006, 8, 4939.
[20] Li, H.; Wang, Y.-Q.; Deng, L. Org. Lett. 2006, 8, 4063.
[21] He, X.; Hu, S.; Liu, K.; Guo, Y.; Xu, J.; Shao, S. Org. Lett. 2006, 8, 333.
[22] Esquivias, J.; Arrayas, R. G.; Carretero, J. C. Angew. Chem., Int. Ed. 2006, 45, 629.
/
| 〈 |
|
〉 |