

合成取代的N—F双苯磺酰亚胺及其与烯醇硅醚的反应比较
收稿日期: 2015-06-12
修回日期: 2015-07-13
网络出版日期: 2015-08-17
基金资助
国家自然科学基金(No. 2132077)资助项目.
Synthesis of Substituted N—F Benzenesulfonimides and Comparison of Their Fluorination Reactivity via Their Reactions with Silylenol Ethers
Received date: 2015-06-12
Revised date: 2015-07-13
Online published: 2015-08-17
Supported by
Project supported by the National Natural Science Foundation of China (No. 2132077).
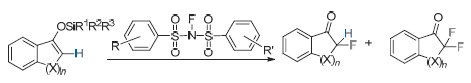
N—F双苯磺酰胺作为一种常用的氟化试剂, 会因为苯环上修饰不同的官能团而影响试剂本身的电子效应, 这些改变会影响到试剂中N—F键的强弱, 也就是试剂的氟化活性, 进而对氟化反应产生比较大的影响. 主要合成了不同取代基的N—F苯磺酰胺试剂, 并且用这些试剂与几种烯醇硅醚进行氟化反应, 以期望找到活性更高的氟化试剂. 从实验结果可以看出, 在众多的不同取代基的N—F苯磺酰胺试剂中, N-氟-4-硝基-4'-氟双苯磺酰胺与N-氟-4-叔丁基-4'-氟双苯磺酰胺的氟化效果优于N-氟双苯磺酰胺(NFSI). 对这两种试剂进行了一系列的底物的拓展, 并且与N-氟苯磺酰胺(NFSI)进行了比较. 实验结果表明, 对于某些底物论这两种试剂的氟化性能比未经修饰的N-氟苯磺酰胺(NFSI)要好.
关键词: 取代基的N—F双苯磺酰胺; 氟化反应; 烯醇硅醚
李青巍 , 陈福利 , 杨先金 . 合成取代的N—F双苯磺酰亚胺及其与烯醇硅醚的反应比较[J]. 有机化学, 2015 , 35(12) : 2604 -2609 . DOI: 10.6023/cjoc201506014
The fluorination reactivity of N—F benzenesulfonimides depended on the strength of N—F bond and was easily affected by substituents on the phenyl of substituted N—F benzenesulfonimides via electronic or steric effect. In this work, N—F benzenesulfonimides with different substituents were synthesized and reacted with silylenol ethers, and the results indicated that utilizing N-fluoro-4-fluoro-4'-nitrobenzenesulfonimide and N-fluoro-4-tert-butyl-4'-fluorobenzenesulfonimide could improve the reactivity or selectivity of fluorination than using N—F benzenesulfonimide in lots of selected experiments.

[1] Shimizu, M.; Hiyama, T. Angew. Chem., Int. Ed. 2005, 44, 214.
[2] Welch, J. T. Tetrahedron 1987, 43, 3123.
[3] Ni, C.; Zhu, L.; Hu, J. Acta Chim. Sinica 2015, 73, 90 (in Chinese). (倪传法, 朱林桂, 胡金波, 化学学报, 2015, 73, 90.)
[4] Zhang, J.; Jin, C.; Zhang, Y. Chin. J. Org. Chem. 2014, 34, 662 (in Chinese). (张霁, 金传飞, 张英俊, 有机化学, 2014, 34, 662.)
[5] Wang, G.; He, X.; Dai, J. Chin. J. Org. Chem. 2014, 34, 837 (in Chinese). (王光祖, 赫侠平, 戴建军, 许华建, 有机化学, 2014, 34, 837.)
[6] Zhang, K.; Xu, X.; Qing, F. Chin. J. Org. Chem. 2015, 35, 556 (in Chinese). (张柯, 徐修华, 卿凤翎, 有机化学, 2015, 35, 556.)
[7] Differding, E.; Lang, R. W. Tetrahedron Lett. 1988, 29, 6087.
[8] Differding, E.; Ofner, H. Synlett 1991, 187.
[9] Davis, F. A.; Zhou, P.; Murphy, C. K. Tetrahedron Lett. 1993, 34, 3971.
[10] Lal, G. S.; Pez, G. P.; Syvret, R. G. Chem. Rev. 1996, 96,1737.
[11] (a) Purrington, S. T.; Kagen, B. S.; Patrick, T. B. Chem. Rev. 1986, 86, 997. (b) Liu, C.-B.; Ma, H.; Nie, J.; Ma, J.-A. Chin. J. Chem. 2012, 30, 47.
[12] Banks, R. E.; Mohialdin-Khaffaf, S. N.; Lal, G. S.; Sharif, I.; Syvret, R. G. J. Chem. Soc., Chem. Commun. 1992, 595.
[13] Umemoto, T.; Fukami, S.; Tomizawa, G.; Harasawa, K.; Kawada, K.; Tomita, K. J. Am. Chem. Soc. 1990, 112, 8563.
[14] Ishimaru, T.; Shibata, N.; Horikawa, T.; Yasuda, N.; Nakamura, S.; Toru, T.; Shiro, M. Angew. Chem., Int. Ed. 2008, 47, 4157.
[15] (a) Greedy, B.; Paris, J. M.; Vidal, T.; Gouverneur, V. Angew.Chem. 2003, 115, 3413. (b) Zhang, R.; Wang, D.; Xu, Q.; Jiang, J.-J.; Shi, M. Chin. J. Chem. 2012, 30, 1295.
[16] Shibatomi, K.; Yamamoto, H. Angew. Chem., Int. Ed. 2008, 47, 5796.
[17] Yasui, H.; Yamamoto, T.; Ishimaru, T.; Fukuzumi, T.; Tokunaga, E.; Akikazu, K.; Shiro, M.; Shibata, N. J. Fluorine Chem. 2011, 132, 222.
[18] Zhu, C.-L.; Maeno, M.; Zhang, F.-G.; Shigehiro, T.; Kagawa, T.; Kawada, K.; Shibata, N.; Ma, J.-A.; Cahard, D. Eur. J. Org. Chem. 2013, 29, 6501.
[19] Shunatona, H. P.; Frh, N.; Wang, Y. M.; Rauniyar, V.; Toste, F. D. Angew. Chem. Int. Ed. 2013, 52, 7724.
[20] Chen, G. L.; Chen, F. L.; Zhang, Y. ; Yang, X. Y.; Yuan, X. M.; Wu, F. H.; Yang, X. J. J. Fluorine Chem. 2012, 133, 155.
[21] Zhang, Y.; Yang, X. J.; Xie, T.; Chen, G. L.; Zhu, W. H.; Zhang, X. Q.; Yang, X. Y.; Wu, X. Y.; He, X. P.; He, H. M. Tetrahedron 2013, 69, 4933.
[22] Wang, F.-J.; Li, J.-L.; Hu, Q.-Y.; Yang, X.-J.; Wu, X.-Y.; He, H.-M. Eur. J. Org. Chem.2014, 17, 3607.
[23] Dykhanov, N. N. Zh. Obshch. Khim. 1959, 29, 3602.
[24] Busto, E.; Gotor-Fernández, V.; Gotor, V. J. Org. Chem. 2012, 77, 4842.
[25] Teare, H.; Robins, G. E.; Arstad, E.; Luthra, S. K.; Gouverneur, V. Chem. Commun. 2007, 2330.
[26] Xu, J.; Hu, Y.; Huang, D.; Wang, K. H.; Xu, C.; Niu, T. Adv. Synth. Catal. 2012, 354, 515.
[27] Cahard, D.; Audouard, C.; Plaquevent, J. C.; Roques, N. Org. Lett. 2000, 2, 3699.
[28] Stavber, S.; Šket, B.; Zajc, B.; Zupan, M. Tetrahedron 1989, 45, 6003.
[29] Zhao, Y.; Pen, Y.; Liu, H.; Yang, Y.; Jiang, Z.; Tang, C. Chem. Eur. J. 2011, 17, 3571.
[30] Stavber, S.; Jereb, M.; Zupan, M. Synthesis 2002, 2609.
/
| 〈 |
|
〉 |