

微波促进苊并咪唑氮杂环卡宾钯化合物催化的水相氰基化反应
收稿日期: 2015-06-24
修回日期: 2015-07-09
网络出版日期: 2015-08-17
基金资助
教育部博士点基金(No. 20130071110032)、上海市带头学科(No. B108)及复旦大学卓学资助项目.
Acenaphthoimidazole N-Heterocyclic Carbene Palladium ComplexesCatalyzed Cyanation Reactions in Aqueous Accelerated byMicrowave Irradiation
Received date: 2015-06-24
Revised date: 2015-07-09
Online published: 2015-08-17
Supported by
Project supported by the Research Fund of Doctoral Program, Ministry of Education (No. 20130071110032), the Shanghai Leading Academic Discipline Project (B108) and the Department of Chemistry Fudan University.
蒋晓军, 申雅靓, 刘泽龙, 吕绪良, 涂涛 . 微波促进苊并咪唑氮杂环卡宾钯化合物催化的水相氰基化反应[J]. 有机化学, 2015 , 35(11) : 2389 -2392 . DOI: 10.6023/cjoc201506029
In the presence of acenaphthoimidazole N-heterocyclic carbene palladium complexes, cyanation of aryl bromides by non-toxic and inexpensive potassium hexacyanoferrate (II) as cyanide source in water has been investigated under microwave conditions. Both electron-donating and electron-withdrawing groups attached to the substrates show unobvious effects on the transformation and produce the corresponding products in good to excellent yields within few minutes, which demonstrate the practicability and environmentally friendliness of the new developed protocol.
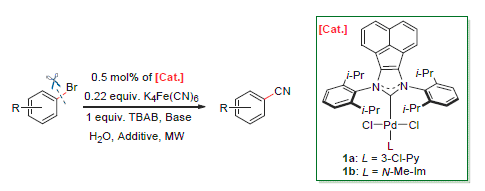
Key words: cyanation; N-heterocyclic carbene; microwave assistant; palladium; aqueous phase
[1] Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances: Syntheses, Patents, Applications, 4th ed., Georg Thieme, Stuttgart, 2001.
[2] (a) Larock, R. C. Comprehensive Organic Transformations, VCH, New York, 1989, p. 819.(b) Grundmann, C. In Houben-Weyl: Methoden der Organischen Chemie, 4th ed., Ed.: Falbe, J., Georg Thieme Verlag, Stuttgart, 1985, Vol. E5, p. 1313.(c) Hagedorn, F.; Gelbke, H.-P. In Ullmanns Encyklopadie der Technischen Chemie, 4th ed., Eds.: Bartholomé, E.; Biekert, E.; Hellmann, H.; Ley, H.; Weigert, W. M.; Weise, E., Verlag Chemie, Weinheim, 1979, Vol. 17, p. 333.(d) Ferri, C. Reaktionen der Organischen Chemie, Georg Thieme Verlag, Stuttgart, 1978, p. 571.(e) Kurtz, P. In Houben Weyl: Methoden Der Organischen Chemie, 4th ed., Ed.: Muller, E., Georg Thieme Verlag, Stuttgart, 1952, Vol. 8, p. 265.
[3] Takagi, K.; Okamoto, T.; Sakakibara, Y.; Oka, S. Chem. Lett. 1973, 471.
[4] (a) Sekiya, A.; Ishikawa, N. Chem. Lett. 1975, 277.(b) Takagi, K.; Okamoto, T.; Sakakibara, Y.; Ohno, A.; Oka, S.; Hayama, N. Bull. Chem. Soc. Jpn. 1975, 48, 3298.(c) Akita, Y.; Shimazaki, M.; Ohta, A. Synthesis. 1981, 974.(d) Prochazka, M.; Siroky, M. Collect. Czech. Chem. Commun. 1983, 48, 1765.(e) Sato, N.; Suzuki, M. J. Heterocycl. Chem. 1987, 24, 1371.(f) Tanji, K.; Higashino, T. Heterocycles 1990, 30, 435.(g) Takagi, K.; Sasaki, K.; Sakakibara, Y. Bull. Chem. Soc. Jpn. 1991, 64, 1118
[5] Dalton, J. R.; Regen, S. L. J. Org. Chem. 1979, 44, 4443.
[6] Chatani, N.; Hanafusa, T. J. Org. Chem. 1986, 51, 4714.
[7] Nair, V.; Purdy, D. F.; Sells, T. B. J. Chem. Soc., Chem. Commun. 1989, 878.
[8] (a) Tschaen, D. M.; Desmond, R.; King, A. O.; Fortin, M. C.; Pipik, B.; King, S.; Verhoeven, T. R. Synth. Commun. 1994, 24, 887.(b) Gundersen, L. L. Acta Chem. Scand. 1996, 50, 58.
[9] (a) Schareina, T.; Zapf, A.; Beller, M. Chem. Commun. 2004, 1388.(b) Merz, V.; Weith, W. Chem. Ber. 1877, 10, 746.
[10] Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. Tetrahedron Lett. 1986, 27, 279.
[11] (a) Li, L.; Pan, Z.; Duan, X.; Liang, Y. Synlett. 2006, 2094.(b) Velmathi, S.; Leadbeate, N. Tetrahedron Lett. 2008, 49, 4693.(c) Chen, G.; Weng, J.; Zheng, Z.; Zhu, X.; Cai, Y.; Cai, J.; Wan, Y. Eur. J. Org. Chem. 2008, 22, 3524.(d) Ren, Y. L.; Wang, W.; Zhao, S.; Tian, X.; Wang, J.; Yin, W.; Cheng, L. Tetrahedron Lett. 2009, 50, 4595.(e) Fang, K.; Wen, W.; Chen, L.; Peng, L. Chin. J. Org. Chem. 2013, 33, 2559 (in Chinese).(柯方, 吴雯, 林晨, 李鹏, 有机化学,2013, 33, 2559.)
[12] Matthew, N.; Hopkinson, C. R.; Michael, S.; Glorius, F. Nat. Chem. 2014, 510, 485.
[13] (a) Tu, T.; Feng, X.; Wang, Z.; Liu, X. Dalton Trans. 2010, 39, 10598.(b) Tu, T.; Mao, H.; Herbert, C.; Xu, M.; Dötzb, K. H. Chem. Commun. 2010, 46, 7796.(c) Tu, T.; Fang, W.; Jiang, J. Chem. Commun. 2011, 47, 12358.(d) Tu, T.; Sun, Z.; Fang, W.; Xu, M.; Zhou, Y. Org. Lett. 2012, 14, 4250.(e) Fang, W.; Deng, Q.; Xu, M.; Tu, T. Org. Lett. 2013, 15, 3678.(f) Jiang, J.; Zhu, H.; Shen, Y.; Tu, T. Org. Chem. Front. 2014, 1, 1172.
[14] Alagiri, K.; Prabhu, K. R. Tetrahedron 2011, 67, 8544.
[15] Zhou, W.; Wang, K.; Wang, J. Adv. Synth. Catal. 2009, 351, 1378.
[16] Grossman, O.; Gelman, D. Org. Lett. 2006, 8, 1189.
[17] Mino, T.; Koizumi, T.; Shibuya, M.; Hirai, K.; Sakamoto, M.; Fujita, T. Heterocycles 2011, 83, 163.
[18] Karthikeyan, J.; Parthasarathy, K.; Cheng, C. Chem. Commun. 2011, 47, 10461.
Qin, W.; Yasuike, S.; Kakusawa, N.; Kurita, J. J. Organomet. Chem. 2008, 693, 2949.
/
| 〈 |
|
〉 |