

不同受体单元的两个聚合物光伏性质
收稿日期: 2015-05-18
修回日期: 2015-07-09
网络出版日期: 2015-08-25
基金资助
河南师范大学青年基金(No. 01026400061)、河南省基础与前沿技术研究计划(No. 1323004100247)、大学生创新(No. 2012011)资助项目.
Photovoltaic Property of Two Polymers Furnished by Different Acceptor Bolcks
Received date: 2015-05-18
Revised date: 2015-07-09
Online published: 2015-08-25
Supported by
Project supported by the Young Scientists Foundation of Henan Normal University (No. 01026400061), the Henan Province Basic and Frontier Technology Research Projects (No. 1323004100247) and the College Students’ Innovative Pilot Scheme (No. 2012011).
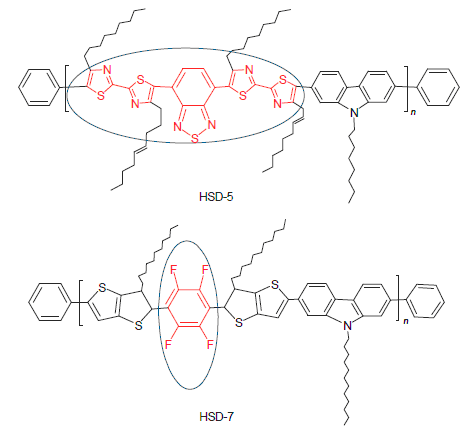
合成了两个不同受体的共轭聚合物联噻唑-苯并噻二唑-咔唑共聚物(HSD-5); 四氟苯-并二噻吩-咔唑共聚物(HSD-7), 研究了其热学、光物理和光伏性质. 由电化学结果显示两个聚合物的带隙分别为2.16和2.53 eV. 用聚合物/ [6,6]-苯基-C71-丁酸甲酯(PC71BM)作为活性层构筑了本体异质结聚合物太阳能电池的能量转换效率分别为0.36%和0.73%. 同时, 研究表明含氟材料由于碳-氟键高度极化改变了聚合物分子间的作用力, 对活性层的形貌产生显著影响; 多元受体单元间较大的扭转角会降低共轭电子离域程度, 不利于分子内因电荷转移对光子的吸收, 拓宽了聚合物的能带隙. 最后, 结合实验结果分析了两种材料制备的器件能量转换效率较低的原因.
秦瑞平 , 耿凡 , 王丹丰 , 姚小静 . 不同受体单元的两个聚合物光伏性质[J]. 有机化学, 2015 , 35(12) : 2583 -2588 . DOI: 10.6023/cjoc201505028
Two conjugated copolymers poly[4-(4,4'-dinonyl-2,2'-bithiazol-5-yl)-7-(4,4'-dinonyl-5'-(9-octyl-9H-carbazol- 2-yl)-2,2'-bithiazol-5-yl)benzo[c][1,2,5]thiadiazole] (HSD-5) and poly[9-octyl-2-(5-(2,3,5,6-tetrafluoro-4-(3-undecylthieno- [3,2-b]thiophen-2-yl)phenyl)-6-undecylthieno[3,2-b]thiophen-2-yl)-9H-carbazole] (HSD-7) were synthesized and their photophysical and photovoltaic properties were studied. The electrochemical characterization results showed that these two polymer possessed wide band-gaps of 2.16 and 2.53 eV, respectively. Solar cell devices based on polymer/PC71BM gave a power conversion efficiency (PCE) of 0.36% and 0.73% for HSD-5 and HSD-7, respectively. For the fluorine containing polymer HSD-7, the strong electronegativity of fluorine induces a strong dipole moment along the C—F bond, which resulted in a strong inter/intramolecular interaction and displayed significant influnce on the morphology of the active layer. Meanwhile, the large torsion angles between the acceptor blocks destroyed the conjugation and the electron delocalization. Finally, the structure-activity relationship was also discussed to understand the reason of the low power conversion efficiency (PCE).

[1] (a) Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Nat. Commun. 2014, 5, 5293/1. (b) Chen, J.-D.; Cui, C.; Li, Y.-Q.; Zhou, L.; Ou, Q.-D.; Li, C.; Li, Y.; Tang, J.-X. Adv. Mater. 2015, 27, 1035.
[2] Meng, Q.-B. Acta Chim. Sinica 2015, 73, 161 (in Chinese). (孟庆波, 化学学报, 2015, 73, 161.)
[3] (a) Qin, R.-P.; Li, W.-W.; Li, C.-H.; Du, C.; Veit, C.; Schleiermacher, H F.; Andersson, M.; Bo Z.-S.; Liu Z.-P.; Inganas, O.; Wuerfel, U.; Zhang, F.-L. J. Am. Chem. Soc. 2009, 131, 14612. (b) Lei, T.; Wang, J.-Y.; Pei, J. Acc. Chem. Res. 2014, 47, 1117. (c) Coughlin, J. E.; Henson, Z. B.; Welch, G. C. Acc. Chem. Res. 2014, 47, 257. (d) Qin, R.-P.; Jiang, Y.-R.; Ma, H; Yang, L; Liu, H.-Z.; Chang, F.-G. J. Appl. Polym. Sci. 2013, 129, 2671. (e) Qin, R.-P.; Song, G.-L.; Jiang, Y.-R.; Bo, Z.-S. Chem. J. Chin. Univ. 2012, 33, 828 (in Chinese). (秦瑞平, 宋桂林, 蒋玉荣, 薄志山, 高等学校化学学报, 2012, 33, 828.) (f) Liu, Z.; Xu, F.; Yan, D.-D. Acta Chim. Sinica 2014, 72, 171 (in Chinese). (刘震, 徐丰, 严大东, 化学学报, 2014, 72, 171.)
[4] (a) Gong, X.; Li, C.; Lu, Z.; Li, G.; Mei, Q.; Fang, T.; Bo, Z.-S. Macromol. Rapid Commun. 2013, 34, 1163. (b) Chen, Y.; Wan, X.; Long, G. Acc. Chem. Res. 2013, 46, 2645. (c) You, J.; Dou, L.; Yoshimura, K. Nat. Commun. 2012, 4, 1446. (d) Fan, X.-C.; Wang, F.; Li, X.-C.; Chen, Y.; Lai, W.-Y.; Huang, W. Chin. J. Org. Chem. 2014, 34, 2027 (in Chinese). (范晓春, 王芳, 李祥春, 陈垚, 赖文勇, 黄维, 有机化学, 2014, 34, 2027.)
[5] (a) Wang, X.; Sun, Y.; Chen, S. Macromolecules 2012, 45, 1208. (b) Uy, R. L.; Price, S. C.; You, W. Macromol. Rapid Commun. 2012, 33, 1162. (c) Li, Y.-F. Acc. Chem. Res. 2012, 45, 723. (d) Du, C.; Li, W.; Li, C. J. Polym. Sci., Part A: Polym. Chem. 2012, 51, 383.
[6] (a) Lei, T.; Wang, J.-Y.; Pei, J. Chem. Mater. 2014, 26,594. (b) Hu, X.-L.; Zuo, L.-J.; Nan, Y.-X. Synth. Met. 2012, 162, 2005.
[7] (a) Brocks, G.; Tol, A. J. Phys. Chem. 1996, 100, 1838. (b) Ajayaghosh, A. Chem. Soc. Rev. 2003, 32, 181.
[8] (a) Shi, Q.; Fan, H.; Liu, Y. Macromolecules 2011, 44, 4230. (b) Shi, Q.; Fan, H.; Liu, Y. J. Phys. Chem. C 2010, 114, 16843.
[9] Zhang, X.; Köhler, M.; Matzger, A. J. Macromolecules 2004, 37, 6306.
[10] Qin, R.-P.; Bo, Z.-S. Macromol. Rapid Commun. 2012, 33, 87.
/
| 〈 |
|
〉 |