

具有5-芳氧甲基(芳胺甲基)链接的3-芳基异噁唑衍生物的合成及其生物活性初探
收稿日期: 2015-05-28
修回日期: 2015-06-22
网络出版日期: 2015-08-25
基金资助
国家自然科学基金(Nos. 21272153, 21072128)资助项目.
Synthesis of Novel 3-Aryl-isoxazoles Bearing 5-Aryloxymethyl or 5-Arylaminomethyl Linkage System as Potential Pesticides
Received date: 2015-05-28
Revised date: 2015-06-22
Online published: 2015-08-25
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21272153, 21072128).
徐姗姗 , 张敏 , 张丽 , 蒋海芳 , 朱宁 , 宋力平 , 邓红梅 . 具有5-芳氧甲基(芳胺甲基)链接的3-芳基异噁唑衍生物的合成及其生物活性初探[J]. 有机化学, 2015 , 35(12) : 2595 -2603 . DOI: 10.6023/cjoc201505047
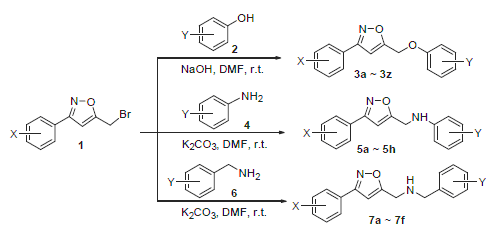
A new class of 3-aryl-5-aryloxymethylisoxazole derivatives and 3-aryl-5-arylaminomethyl (or 5-benzylamino- methyl) isoxazole derivatives were designed and efficiently synthesized by nucleophilic substitution reaction of 3-aryl-5-bro- momethylisoxazole (1) with the corresponding phenols or anilines (or benzyl amines), respectively. All of the target compounds were characterized by 1H NMR, IR, MS and elemental analysis. Besides, a biological activity study was performed to evaluate the mortality of 3-aryl-5-aryloxy-methylisoxazoles (3) towards Aphis crassivora. Particularly, 3-(2-cyanophenyl)-5- (2-chrolophenoxymethyl)isoxazole (3p) demonstrated a moderate biological activity.
[1] Loaiza, P. R.; Quintero, A. R.; Solano, J. D.; Rocha, A. L. Eur. Med. Chem. 2004, 39, 5.
[2] Nakamura, I.; Yamamoto, Y. Chem. Rev. 2004, 104, 2127.
[3] Matthew, P.; Bourbeau, T. R. Org. Lett. 2006, 17, 3679.
[4] Niu, T. F.; Lv, M. F.; Wang, L.; Yi, W. B.; Cai, C. Org. Biomol. Chem. 2013, 11, 1040.
[5] Liu, Q.; Hou, X. Phosphorus, Sulfur Silicon Relat. Elem. 2012, 187, 448.
[6] Kumar, K. A.; Lokeshwari, D. M.; Kumar, G.. V. Intel. J. Pharm. Sci. Drug Res. 2012, 4, 236.
[7] Patela, J.; Malania, M.; Andreib, G.; Balzarinib, J.; Snoeckb, R.; Dholakiyaa, B. Anti-Infect. Agents 2014, 12, 104.
[8] Rashad, A. A.; Ei-Sabbagh, O. I.; Baraka, M. M.; Ibrahim, S. D.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Balzarini, J.; Mostafa, A. Med. Chem. Res. 2010, 19, 1025.
[9] Vicentino, A. R. R.; Carneiro1, V. C.; Amarante, A. M.; Benjamim, C. F.; Aguiar, A. P.; Fantappie, M. R. PLoS One 2012, 7, 49.
[10] Stefan, A. L.; Simona, M.; Martina, D. F. Chem. Med. Chem. 2006, 1, 197.
[11] Akbarzadeh, T.; Rafinejad, A.; Safavi, M.; Pordeli, M.; Shafiee, A.; Foroumadi, A. Arch. Pharm. Chem. Life Sci. 2012, 345, 386.
[12] Yong, J.; Lu, C.; Wu, X. Med. Chem. Commun. 2014, 5, 968.
[13] Kumbhare, R. M.; Kosurkar, U. B.; Janaki, R. M.; Dadmal, T. L.; Pushpavalli, S. N.; Pal-Bhadra, M. Bioorg. Med. Chem. Lett. 2012, 22, 5424.
[14] Nandini, C. P.; Schwarz, J.; Hou, X.; Hoover, D. J.; Xie, L.; O'Donnell, C. J. J. Med. Chem. 2013, 56, 9180.
[15] Yong, J. P.; Lu, C. Z.; Wu, X. Y. Anti-Cancer Agents Med. Chem. 2015, 15, 131.
[16] Kumbhare, R. M.; Dadmal, T. L.; Devi, T. A.; Kumar, D.; Kosurkar, Umesh. B.; Chowdhury, D.; Appalanaidu, K.; Rao, Y. K.; Ramaiah, M. J.; Bhadr, M. P. Med. Chem. Commun. 2014, 5, 1744.
[17] Koufaki, M.; Fotopoulou, T.; Kapetanou, M.; Heropoulos, G. A.; Gonos, E. S.; Chondrogianni, N. Eur. J. Med. Chem. 2014, 83, 508.
[18] Huang, Q. Q.; Mao, J. L.; Wan, B. J.; Wang, Y. H.; Brun, R.; Franzblau, S. G.; Kozikowski, A. P. J. Med. Chem. 2009, 52, 6757.
[19] Pieroni, M.; Lilienkampf, A.; Wang, Y. H.; Wan, B. J; Cho, S. Y.; Franzblau, S. G.; Kozikowski, A. P. Chem. Med. Chem. 2010, 5, 1667.
[20] Shibata, C. JP 2010077090, 2010 [Chem. Abstr. 2010, 152, 429692].
[21] Yang, Y. D.; Zhang, M.; Zhu, Y. W.; Zhang, L.; Xie, Q. Q.; Song L. P.; Deng, H. M. Chin. J. Chem. 2013, 31, 950.
/
| 〈 |
|
〉 |