

新型含4-苯基-5-硫亚基-1,2,4-三唑曼尼希碱的喹唑啉酮类衍生物的设计合成及其生物活性研究
收稿日期: 2015-06-24
修回日期: 2015-08-18
网络出版日期: 2015-09-15
基金资助
国家自然科学基金(No. 21362003)资助项目.
Design, Synthesis and Biological Activities of Novel Quinazolinone Derivatives Bearing 4-Phenyl-5-thioxo-1,2,4-triazole Mannich Bases
Received date: 2015-06-24
Revised date: 2015-08-18
Online published: 2015-09-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21362003).
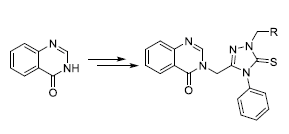
以喹唑啉-4-酮为起始原料, 经五步反应合成了9个新型的含4-苯基-5-硫亚基-1,2,4-三唑曼尼希碱的喹唑啉酮类衍生物6a~6i, 通过1H NMR、13C NMR、IR和元素分析对它们的结构进行了表征, 并用X射线单晶衍射法测定了3-[(1-吗啉甲基-4-苯基-5-硫亚基-4,5-二氢-1H-1,2,4-三唑-3-基)甲基]喹唑啉-4(3H)-酮(6e)的晶体结构. 初步生物活性测试结果表明, 绝大部分该类化合物在200 μg/mL浓度下对水稻白叶枯病菌和柑橘溃疡病菌都表现出了优良的抑制活性; 在50 μg/mL浓度下该类化合物对所测六种真菌都具有一定的抑制活性.
闫柏任, 吕新阳, 杜欢, 鲍小平 . 新型含4-苯基-5-硫亚基-1,2,4-三唑曼尼希碱的喹唑啉酮类衍生物的设计合成及其生物活性研究[J]. 有机化学, 2016 , 36(1) : 207 -212 . DOI: 10.6023/cjoc201506026
Using quinazolin-4-one as the starting compound, nine novel quinazolinone derivatives 6a~6i bearing the 4-phenyl-5- thioxo-1,2,4-triazole Mannich base unit were prepared through a five-step synthetic procedure. The structures of target compounds were characterized by 1H NMR, 13C NMR, IR and elemental analysis. 3-((1-Morpholinomethyl-4-phenyl- 5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)methyl)quinazolin-4(3H)-one was further confirmed by X-ray single crystal diffraction method. The preliminary biological test indicated that almost all of the title compounds had excellent antibacterial activities against Xanthomonas oryzae pv. oryzae and Xanthomonas axonopodis pv. citri at the concentration of 200 μg/mL. Moreover, compounds 6a~6i displayed a certain inhibitory effect against six kinds of tested fungi at the concentration of 50 μg/mL.

[1] Cao, S.-L.; Feng, Y.-P.; Jiang, Y.-Y.; Liu, S.-Y.; Ding, G.-Y.; Li, R.-T. Bioorg. Med. Chem. Lett. 2005, 15, 1915.
[2] Wang, X.; Yin, J.; Shi, L.; Zhang, G.-P.; Song, B.-A. Eur. J. Med. Chem. 2014, 77, 65.
[3] Bartroli, J.; Turmo, E.; Algueró, M.; Boncompte, E.; Vericat, M. L.; Conte, L.; Ramis, J.; Merlos, M.; García-Rafanell, J.; Forn, J. J. Med. Chem. 1998, 41, 1869.
[4] Zhu, S.; Wang, J.; Chandrashekar, G.; Smith, E.; Liu, X.; Zhang, Y. Eur. J. Med. Chem. 2010, 45, 3864.
[5] Kumar, K. S.; Ganguly, S.; Veerasamy, R.; Clercq, E. D. Eur. J. Med. Chem. 2010, 45, 5474.
[6] Wang, X.; Li, P.; Li, Z.-N.; Yin, J.; He, M.; Xue, W.; Chen, Z.-W.; Song, B.-A. J. Agric. Food Chem. 2013, 61, 9575.
[7] Yang, X-H.; Wang, X.; Wu, M.-H. Chin. J. Org. Chem. 2014, 34, 1015 (in Chinese).(杨绪红, 王翔, 吴鸣虎, 有机化学, 2014, 34, 1015.)
[8] Al-Omar, M. A.; Al-Abdullah, E. S.; Shehata, I. A.; Habib, E. E.; Ibrahim, T. M.; El-Emam, A. A. Molecules 2010, 15, 2526.
[9] Plech, T.; Wujec, M.; Majewska, M.; Kosikowska, U.; Malm, A. Med. Chem. Res. 2013, 22, 2531.
[10] Wang, B.-L.; Liu, X.-H.; Zhang, X.-L.; Zhang, J.-F.; Song, H.-B.; Li, Z.-M. Chem. Biol.Drug Des. 2011, 78, 42.
[11] Koparir, M.; Orek, C.; Parlak, A. E.; Söylemez, A.; Koparir, P.; Karatepe, M.; Dastan, S. D. Eur. J. Med. Chem. 2013, 63, 340.
[12] Wang, B.-L.; Shi, Y.-X.; Ma, Y.; Liu, X.-H.; Li, Y.-H.; Song, H.-B.; Li, B.-J.; Li, Z.-M. J. Agric. Food Chem. 2010, 58, 5515.
[13] Alam, M. M.; Nazreen, S.; Haider. S.; Shafi, S.; Yar, M. S.; Hamid, H.; Alam, M. S. Arch. Pharm. Chem. Life Sci. 2012, 345, 203.
[14] Dai, H.; Liu, J.-B.; Zhang, X.; Yu, H.-B.; Qing, X.; Qin, Z.-F.; Wang, T.-T; Fang, J.-X. Chin. J. Org. Chem. 2009, 29, 123 (in Chinese). (戴红, 刘建兵, 张欣, 于海波, 秦雪, 秦振芳, 王婷婷, 方建新, 有机化学, 2009, 29, 123.)
[15] Zhang, M.; Lu, J.-R; Xin, C.-W.; Liu, F.; Wang, J.-J.; Li, H.-J.; Wei, R.-B.; Bao, X.-R. Chin. J. Org. Chem. 2009, 29, 1645 (in Chinese).(张明, 卢俊瑞, 辛春伟, 刘芳, 王菁菁, 李红姬, 魏荣宝, 鲍秀荣, 有机化学, 2009, 29, 1645.)
[16] Sun, J.; Li, D.-D.; Li, J.-R.; Fang, F.; Du, Q.-R.; Qian, Y.; Zhu, H.-L. Org. Biomol. Chem. 2013, 11, 7676.
[17] Mali, J. R.; Pratap, U. R.; Netankar, P. D.; Mane, R. A. Tetrahedron Lett. 2009, 50, 5025.
[18] Kong, F.-B.; Gao, Y.-F.; Chen, X.-L.; Li, G.-L.; Chen, J. Guangxi Agric. Sci. 2006, 37, 148 (in Chinese).(孔凡彬, 高扬帆, 陈锡岭, 李广领, 陈军, 广西农业科学, 2006, 37, 148.)
/
| 〈 |
|
〉 |