

萘氧桥香豆素-双核锌酞菁的合成与性能研究
收稿日期: 2015-08-11
修回日期: 2015-09-02
网络出版日期: 2015-09-15
基金资助
国家自然科学基金(No. 51272239)、山西省自然科学基金(No. 2015011011)和山西省回国留学人员科研(No. 2015-078)资助项目.
Synthesis and Characterization of the Coumarin-Binuclear Zinc Phthalocyanine Formed by Droxynaphthyl Bridge
Received date: 2015-08-11
Revised date: 2015-09-02
Online published: 2015-09-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 51272239), the National Natural Science Foundation of Shanxi Province (No. 2015011011) and the Shanxi Scholarship Council of China (No. 2015-078).
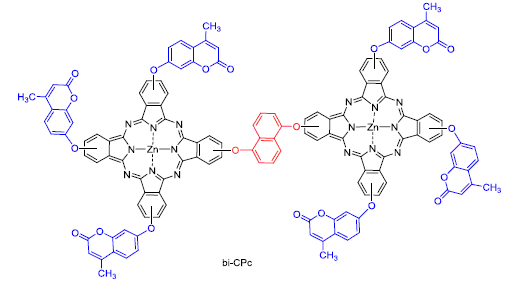
以1,5-二羟基萘、4-硝基邻苯二甲腈、间苯二酚、乙酰乙酸乙酯以及醋酸锌为主要原料, 合成了9(10),16(17),23(24)-三[4-甲基-7-香豆素氧基]-2(3)-(1,5-二氧基萘)双核锌酞菁(bi-CPc). 利用1H NMR、FT-IR、UV-Vis、元素分析、热重分析等手段对前驱体和bi-CPc的结构和性能进行 表征. 结果表明, bi-CPc的紫外可见吸收光谱表现出强烈的π-π*跃迁且在N,N-二甲基甲酰胺(DMF)溶液中以单体形式存在, 并且具有良好的热稳定性. 用循环伏安法研究了bi-CPc的氧化还原行为, 计算其LUMO和HOMO能级与纳米TiO2能级匹配. 通过进一步结构修饰, 有望作为性能良好的光敏染料用于太阳能电池.
曹杰 , 廖超强 , 杨玲 , 张学俊 . 萘氧桥香豆素-双核锌酞菁的合成与性能研究[J]. 有机化学, 2016 , 36(1) : 213 -217 . DOI: 10.6023/cjoc201508013
9(10),16(17),23(24)-Tris[4-methyl-coumarin-7-yloxy]-2(3)-(1,5-naphthalene) binuclear zinc phthalocyanine (bi-CPc) based 1,5-dihydroxynaphthalene, 4-nitro-phthalonitrile, resorcinol, ethyl acetoacetate and zinc acetate on raw material was synthesized. The structures and properties of precursors and bi-CPc were characterized by 1H NMR, FT-IR, UV-Vis, elemental analysis and thermogravimetric analysis. Results showed that strong π-π* transition was demonstrated by UV-Vis absorption spectrum of bi-CPc. At the same time, it existed in a form of monomer in solution of N,N-dimethylformamide (DMF) and possessed excellent thermal stability. Redox behavior of bi-CPc was researched by cyclic voltammetry (CV), and energy level of LUMO and HOMO of bi-CPc matched with energy level of nano-TiO2 after calculating. Through further structural modification, it is expected to use as photosensitive dye with excellent performance for solar cells.

Key words: phthalocyanine; binuclear; coumarin; dye-sensitized solar cells
[1] O'Regan, B.; Grätzel, M. Nature 1991, 353, 737
[2] Grätzel, M. J. Photochem. Photobiol. A 2004, 164, 34
[3] He, J.-J.; Chen, Y.-S.; Wang, T.-T.; Zeng, H.-P. Org. Chem. 2012, 32, 472 (in Chinese).(何俊杰, 陈舒欣, 王婷婷, 曾和平, 有机化学, 2012, 32, 472.)
[4] Hou, Y.-J.; Xie, P.-H.; Zhang, B.-W.; Cao, Y.; Xiao, X.-R.; Wang, W.-B. Inorg. Chem. 1999, 38, 6320.
[5] Horiuchi, T.; Miura, H.; Uchida, S. J. Photochem. Photobiol. A: Chem. 2004, 164, 29.
[6] Hara, K.; Kurashige, M.; Ito, S.; Shinpo, A.; Suga, S.; Sayama, K.; Arakawa, H. Chem. Commun. 2003, 252.
[7] Karthikeyan, C. S.; Peter, K.; Wietasch, H.; Thelakkat, M.; Energy, M. Sol. Cells 2007, 91, 432.
[8] Hara, K.; Sayama, K.; Ohga, Y.; Shinpo; Suga, S.; Arakawa, H. Chem. Commun. 2001, 569.
[9] Kitamura, T.; Ikeda, M.; Shigaki, K.; Inoue, T.; Andenson, N. A.; Ai, X.; Lian, T. Q.; Yanagida, S. Chem. Mater. 2004, 16, 1806.
[10] Hwang, S.; Lee, J. H.; Park, C.; Lee, H.; Kim, C.; Lee, M. H.; Lee, W.; Paka, J.; Kim, K.; Park, N. G.; Kim, C. Chem. Commun. 2007, 4887.
[11] Hara, K.; Miyamoto, K.; Abe, Y.; Yanagida, M. J. Phys. Chem. B 2005, 109, 23776.
[12] Wang, Y.-Q.; Lu, X.; Wei, X.-D.; Wu, W.-J.; Xie, Y.-S. New J. Chem.2014, 38: 3227.
[13] Mathew, S.; Yella, A; Gao, P.; Humphry-Baker, R. Nat. Chem. 2014, 6, 242.
[14] Takuro, L.; Hirotaka, N.; Naruhiko, M.; Matthew, J. G.; Shogo, M.; Mutsumi, K. Chem. Commun. 2014, 50, 1941.
[15] Mao, L.-J.; Tan, Q.-L.; Xing, G.-Q.; Han, M.-L.; Zhang, X.-J. Chin. J. Org. Chem. 2012, 32, 2315 (in Chinese).(毛利军, 谭青龙, 新冠琼, 韩明亮, 张学俊, 有机化学, 2012, 32, 2315.)
[16] Kc, C. C.; Stranius, K.; Dsouza, P. J. Chem. Phys. 2013, 117, 763.
[17] Camur, M.; Ahsen, V.; Durmus, M. Photochem. Photobiol. A: Chem. 2011, 219, 217.
[18] Han, L.; Zhou, X.; Ye, Q.; LI, J.-Y.; Gao, J.-R. Chin. J. Org. Chem.2013, 33, 1000 (in Chinese).(韩亮, 周雪, 叶青, 李郁绵, 高建荣, 有机化学, 2013, 33, 1000.)
[19] Peng, Y.-R.; Huang, F.-H. Strait Pharm. J. 2003, 15, 53 (in Chinese). (彭亦如, 黄风华, 海峡药学, 2003, 15, 53.)
[20] Han, M.-L. M.S. Thesis, North University of China, Taiyuan, 2015 (in Chinese).(韩明亮, 硕士论文, 中北大学, 太原, 2015.)
[21] Leeuw, D. M.; Simenon, M. M. J.; Brown, A. R. Synth. Met. 1997, 87, 53.
/
| 〈 |
|
〉 |