

微波技术在钯催化Suzuki-Miyaura交叉偶联反应中的应用研究进展
收稿日期: 2015-07-12
修回日期: 2015-08-24
网络出版日期: 2015-09-06
基金资助
四川省科技支撑计划基金(No. 2015NZ0033)和西南民族大学中央高校基本科研业务费科学基金(No. 12NZYTH03)资助项目.
Advance on Applications of Microwave Technique in Palladium- Catalyzed Suzuki-Miyaura Cross-Coupling Reaction
Received date: 2015-07-12
Revised date: 2015-08-24
Online published: 2015-09-06
Supported by
Project supported by the Science and Technology Department of Sichuan Province (No. 2015NZ0033) and the Fundamental Research Funds for the Central Universities, Southwest University for Nationalities (No. 12NZYTH03).
与传统的加热方图式相比, 微波加热具有加热速度快、热效率高、节约能源、洁净、操作简单等优点, 已成为重要的有机合成工具之一. 钯催化的Suzuki-Miyaura交叉偶联反应提供了一种合成各种联芳烃的温和方法, 具有较好的选择性, 受到了合成化学家的广泛关注. 综述了近年来微波技术在钯催化的Suzuki-Miyaura交叉偶联反应中的应用研究进展, 包括多种反应体系, 并对其在天然产物和生物活性分子合成中的应用作简要概述.
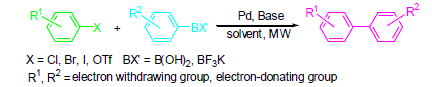
关键词: 微波技术; 钯; Suzuki-Miyaura交叉偶联反应; 催化
李清寒 , 丁勇 , 张刚 , 张震 , 莫松 . 微波技术在钯催化Suzuki-Miyaura交叉偶联反应中的应用研究进展[J]. 有机化学, 2016 , 36(1) : 83 -104 . DOI: 10.6023/cjoc201507008
Compared with conventional heating, microwave heating is one of the most useful tools in organic synthesis because of its obviously advantages of fast heating, thermal efficiency, saving energy, clean, and easy operation. Palladium catalyzed Suzuki-Miyaura cross-coupling reaction could tolerate a broad range of functional groups with high stereoselectivity to provide a mild method in preparation of kinds of substituted biaryls. In this paper, the recent research results about the microwave technique applied in Suzuki-Miyaura cross-coupling reaction are reviewed, involving various reaction systems. The applications of the methodology on the synthesis of natural products and bioactive molecules have also been introduced.
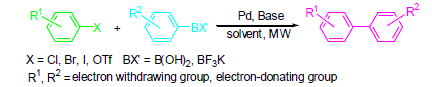
[1] (a) Suzuki, A. J. Organomet. Chem. 1999, 576, 147. (b) Miyaura, N. Top. Curr. Chem. 2002, 219, 11.(c) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457. (d) Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359. (e) Kotha, S.; Lahiri, K.; Kashinath, D. Tetrahedron 2002, 58, 9633. (f) Littke, A. F.; Fu, G. C. Angew. Chem., Int. Ed. 2002, 41, 4176. (g) Miyaura, N. Top. Curr. Chem. 2002, 219, 11.(h) Bellina, F.; Carpita, A.; Rossi, R. Synthesis 2004, 2419. (i) Christmann, U.; Vilar, R. Angew. Chem., Int. Ed. 2005, 44, 366. (j) Alonso, F.; Beletskaya, I. P.; Yus, M. Tetrahedron 2008, 64, 3047.(k) de Meijere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions, Vol. 2, Wiley-VCH, Weinheim, 2004.(l) Ackermann, L. Modern Arylation Methods, Wiley-VCH, Weinheim, 2009.(m) Shen, X.; Jones, G. O.; Watson, D. A.; Bhayana, B.; Buchwald, S. L. J. Am. Chem. Soc. 2010, 132, 11278.(n) Lundin, P. M.; Fu, G. C. J. Am. Chem. Soc. 2010, 132, 11027.(o) Li, W.-Y.; Zhao, D.-M.; Xiong, X. Q.; Ma, Q. Q.; Cheng, M. S. Chin. J. Org. Chem. 2011, 31, 784 (in Chinese).(李文燕, 赵冬梅, 熊绪琼, 马倩倩, 程卯生, 有机化学, 2011, 31, 784.) (p) Li, Q. H.; Ding, Y.; Yang, X. J. Chin. Chem. Lett. 2014, 25, 1296. (q) Li, Q. H.; Ding, Y.; Huang, N. W. Chin. Chem. Lett. 2014, 25(11), 1469.
[2] Xu, G. Q.; Zhao, Q.; Tang, W. J. Chin. J. Org. Chem. 2014, 34, 1919 (in Chinese).(徐广庆, 赵庆, 汤文军, 有机化学, 2014, 34, 1919.)
[3] Zhao, X. W.; Cui, Y. C. Prog. Chem. 2006, 18, 1652 (in Chinese). (赵晓伟, 崔元臣, 化学进展, 2006, 18, 1652.)
[4] Bellina, F.; Carpita, A.; Rossi, R. Synthesis 2004, 2419.
[5] For a discussion, see (a) Grushin, V. V.; Alper, H. In Activation of Unreactive Bonds and Organic Synthesis, Ed.: Murai, S., Springer, Berlin, 1999, p. 193.(b) Grushin, V. V.; Alper, H. Chem. Rev. 1994, 94, 1047.
[6] Polshettiwar, V.; Decottignies, A.; Len, C.; Fihri, A. ChemSusChem 2010, 3, 502.
[7] Fihri, A.; Meunier, P.; Hierso, J. C. Coord. Chem. Rev. 2007, 251, 2017.
[8] Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792.
[9] Liu, L.; Liu, C.; Jin, M. Z. Chin. J. Org. Chem. 2012, 32, 860 (in Chinese).(刘宁, 刘春, 金子林, 有机化学, 2012, 32, 860.)
[10] Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. Tetrahedron Lett. 1986, 27, 279.
[11] Giguere, R. J.; Bray, T. L.; Duncan, S. M.; Majetich, G. Tetrahedron Lett. 1986, 27(41), 4945.
[12] Kappe, C. O. Angew. Chem., Int. Ed. 2004, 43, 6250.
[13] Leadbeater, N. E. Chem. Commun. 2005, 23, 2881.
[14] Dallinger, D.; Kappe, C. O. Chem. Rev. 2007, 107, 2563.
[15] Larhed, M.; Hallberg, A. J. Org. Chem. 1996, 61, 9582.
[16] Blettner. C. G.; König, W. A.; Stenzel, W.; Schotten, T. J. Org. Chem. 1999, 64(11), 3885.
[17] Namboodiri, V. V.; Varma, R. S. Green Chem. 2001, 3(3), 146.
[18] Leadbeater, N. E.; Marco, M. Org. Lett. 2002, 4, 2973.
[19] Leadbeater, N. E.; Marco, M. J. Org. Chem. 2003, 68, 888.
[20] Leadbeater, N. E.; Williams, V. A.; Barnard, T. M.; Collins Jr, M. J. Org. Process Res. Dev. 2006, 10, 833.
[21] Bedford, R. B.; Buts, C. P.; Hurst, T. E.; Lidström, P. Adv. Synth. Catal. 2004, 346, 1627.
[22] Anderson, K. W.; Buchwald, S. L. Angew. Chem., Int. Ed. 2005, 44, 6173.
[23] Genov, M.; Almorín, A.; Espinet, P. Tetrahedron: Asymmetry 2007, 18, 625.
[24] Zhang, Y. Q. J. Chem. Res. 2013, 375.
[25] Kabalka, G. W.; Pagni, R. M.; Wang, L.; Namboodiri, V.; Hair, C. M. Green Chem. 2000, 3, 120.
[26] Kabalka, G. W.; Wang, L.; Pagni, R. M.; Maxwell, H. C.; Vasudevan, N. Synthesis 2003, 217.
[27] Villemin, D.; Caillot, F. Tetrahedron Lett. 2001, 42, 639.
[28] Basu, B.; Das, P.; Bhuiyan, M. M. H.; Jha, S. Tetrahedron Lett. 2003, 44(19), 3817.
[29] Melucci, M.; Barbarella, G.; Sotgiu, G. J. Org. Chem. 2002, 67, 8877.
[30] Sotgiu, G.; Zambianchi, M.; Barbarella, G.; Botta, C. Tetrahedron 2002, 58, 2245.
[31] Melucci, M.; Barbarella, G.; Zambianchi, M.; Pietro, P. D.; Bongini, A. J. Org. Chem. 2004, 69, 4821.
[32] Saha, P.; Naskar, S.; Paira, P.; Hazra, A.; Sahu, K. B.; Paira, R.; Banerjee, S.; Mondal, N. B. Green Chem. 2009, 11, 931.
[33] Cargill, M. R.; Sandford, G.; Tadeusiak, A. J.; Yufit, D. S.; Howard, J. A. K.; Kilickiran, P.; Nelles, G. J. Org. Chem. 2010, 75, 5860.
[34] Gao, F. F.; Liu, F. J.; Lin, C.; Li, W. T.; Wang, S. G.; Qi, C. Z. Catal. Commun. 2013, 5, 27.
[35] Wang, Y.; Sauer, D. R. Org. Lett. 2004, 6, 2793.
[36] Bai, L.; Zhang, Y. M.; Wang, J. X. QSAR Comb. Sci. 2004, 23, 875.
[37] Liu, Y. B.; Khemtong, C.; Hu, J. Chem. Commun. 2004, 398.
[38] Hu, J.; Liu, Y. B. Langmuir 2005, 21, 2121.
[39] Sharma, A. K.; Gowdahalli, K.; Krzeminski, J.; Amin, S. J. Org. Chem. 2007, 72, 8987.
[40] De Souza, A. L. F.; Da Silva, L. C.; Oliveira, B. L.; Antunes, O. A. C. Tetrahedron Lett. 2008, 49, 3895.
[41] Moussa, S.; Siamaki, A. R.; Gupton, B. F.; El-Shall, M. S. ACS Catal. 2012, 2, 145.
[42] Camp, J. E.; Dunsford, J. J.; Cannons, E. P.; Restorick, W. J.; Gadzhieva, A.; Fay, M. W.; Smith, R. J. ACS Sustainable Chem. Eng. 2014, 2, 500.
[43] Gómez-Martínez, M.; Buxaderas, E.; Pastor, I. M.; Alonso, D. A. J. Mol. Catal. A: Chem. 2015, 404~405, 1.
[44] Heidenreich, R. G.; Köhler, K.; Krauter, J. G. E.; Pietsch, J. Synlett 2002, 7, 1118.
[45] Lu, G.; Franzén, R.; Zhang, Q.; Xu, Y. J. Tetrahedron Lett. 2005, 46, 4255.
[46] Arvela, R. K.; Leadbeater, N. E. Org. Lett. 2005, 7, 2101.
[47] Schmidt, B.; Riemer, M.; Karras, M. J. Org. Chem. 2013, 78, 8680.
[48] Schmidt, B.; Riemer, M. J. Org. Chem. 2014, 79, 4104.
[49] Schmidt, B.; Riemer, M. Eur. J. Org. Chem. 2015, 17, 3760.
[50] Al-Amin, M.; Akimoto, M.; Tameno, T.; Ohki, Y.; Takahashi, N.; Hoshiya, N.; Shuto, S.; Arisawa, M. Green Chem. 2013, 15, 1142.
[51] Navarro, O.; Kaur, H.; Mahjoor, P.; Nolan, S. P. J. Org. Chem. 2004, 69, 3173.
[52] Nun, P.; Martinez, J.; Lamaty, F. Synlett 2009, 1761.
[53] Y?lmaz, Ü.; Küçükbay, H.; ?ireci, N.; Akkurt, M.; Günal, S.; Durmaz, R.; Tahir, M. N. Appl. Organomet. Chem. 2011, 25, 366.
[54] Miao, G. B.; Ye, P.; Yu, L. B.; Baldino, C. M. J. Org. Chem. 2005, 70, 2332.
[55] Solodenko, W.; Schön, U.; Messinger, J.; Glinschert, A.; Kirschning, A. Synlett 2004, 1699.
[56] Kopylovich, M. N.; Lasri, J.; Guedes da Silva, M. F. C.; Pombeiro, A. J. L. Dalton Trans. 2009, 16, 3074.
[57] Cívicos, J. F.; Alonso, D. A.; Nájera, C. Adv. Synth. Catal. 2011, 353, 1683.
[58] Dawood, K. M. Tetrahedron 2007, 63, 9642.
[59] Kostas, I. D.; Heropoulos, G. A.; Kovala-Demertzi, D.; Yadav, P. N.; Jasinski, J. P.; Demertzis, M. A.; Andreadaki, F. J.; Vo-Thanh, G.; Petit, A.; Loupy, A. Tetrahedron Lett. 2006, 47, 4403.
[60] Villemin, D.; Escalonilla, M. J. G.; Saint-Clair, J. F. Tetrahedron Lett. 2001, 42(4), 635.
[61] Qu, G. R.; Xin, P. Y.; Niu, H. Y.; Jin, X.; Guo, X. T.; Yang, X. N.; Guo, H. M. Tetrahedron 2011, 67, 9099.
[62] Zhang, W.; Chen, C. H. T.; Lu, Y. M.; Nagashima, T. Org. Lett. 2004, 6, 1473.
[63] Cívicos, J. F.; Alonso, D. A.; Nájera, C. Adv. Synth. Catal. 2012, 354, 2771.
[64] Seganish, W. M.; DeShong, P. J. Org. Chem. 2004, 69, 1137.
[65] Seganish, W. M.; DeShong, P. Org. Lett. 2004, 6, 4379.
[66] Hadebe, S. W.; Sithebe, S.; Robinson, R. S. Tetrahedron 2011, 67, 4277.
[67] Kabalka, G. W.; Al Masum, M. Tetrahedron Lett. 2005, 46, 6329.
[68] Arvela, R. K.; Leadbeater, N. E.; Mack, T. L.; Kormos, C. M. Tetrahedron Lett. 2006, 47, 217.
[69] Kabalka, G. W.; Zhou, L. L.; Naravane, A. Tetrahedron Lett. 2006, 47, 6887.
[70] Harker, R. L.; Crouch, R. D. Synthesis 2007, 25.
[71] Ahn, H. R.; Cho, Y. A.; Kim, D. S.; Chin, J. G.; Gyoung, Y. S.; Lee, S.; Kang, H.; Ham, J. Org. Lett. 2009, 11, 361.
[72] Kim, D. S.; Ham, J. Org. Lett. 2010, 12, 1092.
[73] Nehls, B. S.; Asawapirom, U.; Füldner, S.; Preis, E.; Farrell, T.; Scherf, U. Adv. Funct. Mater. 2004, 14, 352.
[74] Nehls, B. S.; Fldner, S.; Preis, E.; Farrell, T.; Scherf, U. Macromolecules 2005, 38, 687.
[75] Glasnov, T. N.; Stadlbauer, W.; Kappe, C. O. J. Org. Chem. 2005, 70, 3864.
[76] Hashim, J.; Glasnov, T. N.; Kremsner, J. M.; Kappe, C. O. J. Org. Chem. 2006, 71, 1707.
[77] Mathews, C. J.; Taylor, J.; Tyte, M. J.; Worthington, P. A. Synlett 2005, 538.
[78] Song, Y. S.; Kim, B. T.; Heo, J. N. Tetrahedron Lett. 2005, 46, 5987.
[79] Song, Y. S.; Lee, Y. J.; Kim, B. T.; Heo, J. N. Tetrahedron Lett. 2006, 47, 7427.
[80] Heo, Y.; Song, Y. S.; Kim, B. T.; Heo, J. N. Tetrahedron Lett. 2006, 47, 3091.
[81] Arimitsu, S.; Jacobsen, J. M.; Hammond, G. B. J. Org. Chem. 2008, 73, 2886.
[82] Cao, P.; Qu, J. Y.; Burton, G.; Rivero, R. A. J. Org. Chem. 2008, 73, 7204.
[83] Crozet, M. D.; Zink, L.; Remusat, V.; Curti, C.; Vanelle, P. Synthesis 2009, 3150.
[84] Cohen, A.; Crozet, M. D.; Rathelot, P.; Vanelle, P. Green Chem. 2009, 11, 1736.
[85] St. Jean, Jr., D. J.; Poon, S. F.; Schwarzbach, J. L. Org. Lett. 2007, 9, 4893.
[86] Vishnumurthy, K.; Makriyannis, A. J. Comb. Chem. 2010, 12, 664.
[87] Kabri, Y.; Gellis, A.; Vanelle, P. Eur. J. Org. Chem. 2009, 4059.
[88] Kabri, Y.; Crozet, M. D.; Terme, T.; Vanelle, P. Eur. J. Org. Chem. 2015, 3806.
[89] Appukkuttan, P.; Dehaen, W.; Van der Eycken, E. Org. Lett. 2005, 7, 2723.
[90] Appukkuttan, P.; Dehaen, W.; Van der Eycken, E. Chem. Eur. J. 2007, 13, 6452.
[91] Lépine, R.; Zhu, J. Org. Lett. 2005, 7, 2981.
[92] Wannberg, J.; Sabnis, Y. A.; Vrang, L.; Samuelsson, B.; Karlén, A.; Hallberg, A.; Larhed, M. Bioorg. Med. Chem. 2006, 14, 5303.
[93] Ng-Choi, I.; Soler, M.; Cerezo, V.; Badosa, E.; Montesinos, E.; Planas, M.; Feliu, L. Eur. J. Org. Chem. 2012, 4321.
[94] Benali, O.; Deal, M.; Farrant, E.; Tapolczay, D.; Wheeler, R. Org. Process Res. Dev. 2008, 12, 1007.
[95] De Clercq, E. Nat. Rev. Drug Discovery2002, 1, 13.
[96] De Clercq, E. Antiviral Res. 2005, 67, 56.
[97] Capek, P.; Pohl, R.; Hocek, M. Org. Biomol. Chem. 2006, 4, 2278.
[98] Western, E. C.; Shaughnessy, K. H. J. Org. Chem. 2005, 70, 6378.
[99] Western, E. C.; Daft, J. F.; Johnson, E. M.; Gannett, P. M.; Shaughnessy, K. H. J. Org. Chem. 2003, 68, 6767.
[100] Zhu, R.; Qu, F.; Quéléver, G.; Peng, L. Tetrahedron Lett. 2007, 48, 2389.
[101] Gallagher-Duval, S.; Hervé, G.; Sartori, G.; Enderlin, G.; Len, C. New J. Chem. 2013, 37, 1989.
[102] Lussier, T.; Hervé, G.; Enderlin, G.; Len, C. RSC Adv. 2014, 4, 46218.
[103] Spencer, J.; Baltus, C. B.; Patel, H.; Press, N. J.; Callear, S. K.; Male, L.; Coles. S. J. ACS Comb. Sci. 2011, 13, 24.
[104] Arisawa, M.; Sato, T.; Hoshiya, N.; Al-Amin, M.; Kogami, Y.; Shuto, S. ACS Comb. Sci. 2014, 16, 215.
[105] Nakagawa, K.; Hisamatsu, Y.; Moromizato, S.; Kohno, M.; Aoki, S. Inorg. Chem. 2014, 53, 409.
[106] Konda, V.; Rydfjord, J.; Sävmarker, J.; Larhed, M. Org. Process Res. Dev. 2014, 18, 1413.
[107] (a) Baghbanzadeh, M.; Pilger, C.; Kappe, C. O. J. Org. Chem. 2011, 76, 1507; (b)Singh, N.; Singh, R.; Raghuvanshi, D. S.; Singh, K. N. Org. Lett. 2013, 15, 5874; (c) Singh, K. B.; Appukkuttan, P.; Claerhout, S.; Parmar, V. S.; Der Eycken, E. V. Org. Lett. 2006, 8, 1863.
/
| 〈 |
|
〉 |