

对映纯3,4-二氢嘧啶酮衍生物的合成研究进展
收稿日期: 2015-07-01
修回日期: 2015-09-14
网络出版日期: 2015-10-13
基金资助
国家自然科学基金(Nos. 21265009, 21362032)资助项目.
Progress on the Synthesis of Enantiomerically Pure 3,4-Dihydropyrimidin-2-one Derivatives
Received date: 2015-07-01
Revised date: 2015-09-14
Online published: 2015-10-13
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21265009, 21362032).
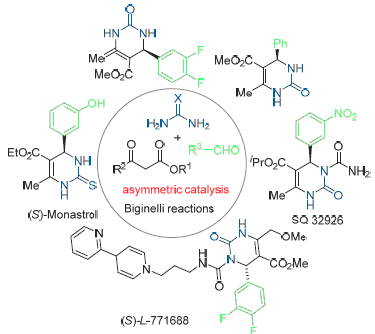
3,4-二氢嘧啶酮本身是不对称分子, 但绝大多数Biginelli反应得到的都是外消旋的3,4-二氢嘧啶酮类化合物. 大量研究表明3,4-二氢嘧啶酮的C(4)立体中心的绝对构型对其生物活性有重要影响. 主要归纳了获得对映纯3,4-二氢嘧啶酮类化合物的新发展, 特别是不对称催化Biginelli反应合成此类化合物的文献方法.
关键词: 对映纯3,4-二氢嘧啶酮; 不对称催化; Biginelli反应; 多组分反应
饶红红 , 权正军 , 白林 , 叶鹤琳 . 对映纯3,4-二氢嘧啶酮衍生物的合成研究进展[J]. 有机化学, 2016 , 36(2) : 283 -296 . DOI: 10.6023/cjoc201507001
3,4-Dihydropyrimidinethiones are chiral molecules, however, only racemic products are isolated in the most reported Biginelli reactions. It has been proved that the absolute configuration of the C(4) stereogenic center has significant influence on the biological activity. The development in the accessing of optically active 3,4-dihydropyrimidinethiones focusing on the recent advances in the asymmetric catalytic Biginelli reactions is summarized.

[1] Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360.
[2] Atwal, K. S.; Rovnyak, G. C.; Schwartz, J.; Moreland, S.; Hedberg, A.; Gougoutas, J. Z.; Malley, M. F.; Floyd, D. M. J. Med. Chem. 1990, 33, 1510.
[3] (a) Rovnyak, G. C.; Atwal, K. S.; Hedberg, A.; Kimball, S. D.; Moreland, S.; Gougoutas, J .Z.; O'Reilly, B. C.; Schwartz, J.; Malley, M. F. J. Med. Chem. 1992, 35, 3254. (b) Deres, K.; Schroder, C. H.; Paessens, A.; Goldmann, S.; Hacker, H. J.; Weber, O.; Kraemer, T.; Niewoehner, U.; Pleiss, U.; Stoltefuss, J.; Graef, E.; Koletzki, D.; Masantschek, R. N. A.; Reimann, A.; Jaeger, R.; Groâ, R.; Beckermann, B.; Schlemmer, K.-H.; Haebich, D.; Rubsamen-Waigmann, H. Science 2003, 299, 893. (d) Kappe, C. O. Eur. J. Med. Chem. 2000, 35, 1043. (c) Lewis, R. W.; Mabry, J.; Polisar, J. G.; Eagen, K. P.; Ganem, B.; Hess, G. P. Biochemistry 2010, 49, 4841. (d) Wan, J.-P.; Pan, Y. Mini-Rev. Med. Chem. 2012, 12, 337.
[4] Hurst, E. W.; Ann. N. Y. Ann. NY Acad. Sci. 1962, 98, 275.
[5] (a) Kappe, C. O. Tetrahedron 1993, 49, 6937. (b) Kappe, C. O. Acc. Chem. Res. 2000, 33, 879. (c) Wan, J.-P.; Liu, Y. Synthesis 2010, 2010, 3943.
[6] Atwal, K. S.; Swanson, B. N.; Unger, S. E.; Floyd, D. M.; Moreland, S.; Hedberg, A.; O'Reilly, B. C. J. Med. Chem. 1991, 34, 806.
[7] Barrow, J. C.; Nantermet, P. G.; Selnick, H. G.; Glass, K. L.; Rittle, K. E.; Gilbert, K. F.; Steele, T. G.; Homnick, C. F.; Freidinger, R. M.; Ransom, R. W.; Kling, P.; Reiss, D.; Broten, T. P.; Schorn, T. W.; Chang, R. S. L.; O′Malley, S. S.; Olah, T. V.; Ellis, J. D.; Barrish, A.; Kassahun, K.; Leppert, P.; Nagarathnam, D.; Forray, C. J. Med. Chem. 2000, 43, 2703.
[8] (a) Maliga, Z.; Kapoor, T. M.; Mitchison, T. J. Chem. Biol. 2002, 9, 989. (b) Debonis, S.; Simorre, J. P.; Crevel, I.; Lebeau, L.; Skoufias, D. A.; Blangy, A.; Ebel, C.; Gans, P.; Cross, R.; Hackney, D. D.; Wade, R. H.; Kozielski, F. Biochemistry 2003, 42, 338.
[9] (a) Du, B.-X.; Quan, Z.-J.; Da, Y.-X.; Zhang, Z.; Wang, X.-C. Adv. Synth. Catal. 2015, 357, 1270. (b) Quan, Z.-J.; Lv,Y.; Jing, F.-Q.; Jia, X.-D.; Huo, C.-D.; Wang, X.-C. Adv. Synth. Catal. 2014, 356, 325. (c) Quan, Z.-J.; Jing, F.-Q.; Zhang, Z.; Da, Y.-X.; Wang, X.-C. Eur. J. Org. Chem. 2013, 2013, 7175. (d) Quan, Z.-J.; Lv, Y.; Wang, Z.-J.; Zhang, Z.; Da, Y.-X.; Wang, X.-C. Tetrahedron Lett. 2013, 54, 1884. (e) Quan, Z.-J.; Zhang, Z.; Da, Y.-X.; Wang, X.-C. Chin. J. Org. Chem. 2009, 29, 876 (in Chinese). (权正军, 张彰, 达玉霞, 王喜存, 有机化学, 2009, 29, 876). (f) Wang, X. C.; Quan, Z. J.; Wang, F.; Wang, M. G.; Zhang, Z.; Li, Z. Synth. Commun. 2006, 36, 451.
[10] (a) Gong, L. Z.; Chen, X. H.; Xu, X.-Y. Chem. Eur. J. 2007, 13, 8920. (b) Heravi, M. M.; Asadi, S.; Lashkariani, B. M. Mol. Diversity 2013, 17, 389. (c) Wan, J.-P.; Lin, F.; Liu, Y. Curr. Org. Chem. 2014, 18, 687.
[11] Atwal, K. S.; Rovnyak, G. C.; Kimball, S. D.; Floyd, D. M.; Moreland, S.; Swanson, B. N.; Gougoutas, J. Z.; Schwartz, J.; Smillie, K. M.; Malley, M. F. J. Med. Chem. 1990, 33, 2629.
[12] Dondoni, A.; Massi, A.; Sabbatini, S. Tetrahedron Lett. 2002, 43, 5913.
[13] (a) Chartrain, C. M.; Ikemoto, N.; Taylor, C. S. WO 9907695, 1999 [Chem. Abstr. 1999, 130, 182478]. (b) Sidler, D. R.; Barta, N.; Li, W.; Hu, E.; Matty, L.; Ikemoto, N.; Campbell, J. S.; Chartrain, M.; Gbewonyo, K.; Boyd, R.; Corley, E. G.; Ball, R. G.; Larsen, R. D.; Reider, P. J. Can. J. Chem. 2002, 80, 646.
[14] Prasad, A. K.; Mukherjee, C.; Singh, S. K.; Brahma, R.; Singh, R.; Saxena, R. K.; Olsen, C. E.; Parmar, V. S. J. Mol. Catal. B: Enzyme 2006, 40, 93.
[15] Dondoni, A.; Massi, A.; Minghini, E.; Sabbatini, S.; Bertolasi, V. J. Org. Chem. 2003, 68, 6172.
[16] Dondoni, A.; Massi, A. Acc. Chem. Res. 2006, 39, 451.
[17] Kappe, C. O.; Uray, G.; Roschger, P.; Lindner, W.; Kratky, C.; Keller, W. Tetrahedron 1992, 48, 5473.
[18] Lou, S.; Taoka, B. M.; Ting, A.; Schaus, S. E. J. Am. Chem. Soc. 2005, 127, 11256.
[19] Lou, S.; Dai, P.; Schaus, S. E. J. Org. Chem. 2007, 72, 9998.
[20] Goss, J. M.; Schaus, S. E. J. Org. Chem. 2008, 73, 7651.
[21] Muñoz-Muñiz, O.; Juaristi, E. ARKIVOC 2003, xi, 16.
[22] Huang, Y.; Yang, F.; Zhu, C. J. Am. Chem. Soc. 2005, 127, 16386.
[23] Cai, Y;-F.; Yang, H.-M.; Li, L.; Jiang, K.-Z.; Lai, G.-Q.; Jiang, J.-X.; Xu, L.-W. Eur. J. Org. Chem. 2010, 2010, 4986.
[24] Karthikeyan, P.; Aswar, S. A.; Muskawar, P. N.; Bhagat, P. R.; Kumar, S. S. J. Organomet. Chem. 2013, 723, 154.
[25] (a) Stephen, J. C. Chem. Eur. J. 2006, 12, 5418. (b) Phillips A. M. F. Eur. J. Org. Chem. 2014, 2014, 7291.
[26] Chen, X.-H.; Xu, X.-Y.; Liu, H.; Cun, L.-F.; Gong, L.-Z. J. Am. Chem. Soc. 2006, 128, 14802.
[27] (a) Li, N.; Chen, X.-H.; Song, J.; Luo, S.-W.; Fan, W.; Gong, L.-Z. J. Am. Chem. Soc. 2009, 131, 15301. (b) Yu, J.; Shi, F.; Gong, L.-Z. Acc. Chem. Res. 2011, 44, 1156.
[28] Xu, F.; Huang, D.; Lin, X.; Wang, Y. Org. Biomol. Chem. 2012, 10, 4467.
[29] González-Olvera, R.; Demare, P.; Regla, I.; Juaristi, E. ARKIVOC 2008, vi, 61.
[30] Xin, J.; Chang, L.; Hou, Z.; Shang, D.; Liu, X.; Feng, X. Chem. Eur. J. 2008, 14, 3177.
[31] Li, Z. Y.; Xing, H. J.; Huang, G. L.; Sun, X. Q.; Jiang, J. L.; Wang, L. Y. Sci. China Chem. 2011, 54, 1726.
[32] Saha, S.; Moorthy, J. N. J. Org. Chem. 2011, 76, 396.
[33] Wu, Y.-Y.; Chai, Z.; Liu, X.-Y.; Zhao, G.; Wang, S.-W. Eur. J. Org. Chem. 2009, 904.
[34] Sohn, J.-H.; Choi, H.-M.; Lee, S.; Joung, S.; Lee, H.-Y. Eur. J. Org. Chem. 2009, 3858.
[35] (a) List, B.; Lerner, R. A.; Barbas, C. F. J. Am. Chem. Soc. 2000, 122, 2395. (b) Ahrendt, K. A.; Borths, C. J.; MacMillan, D. W. C. J. Am. Chem. Soc. 2000, 122, 4243. (c) Yang, D. Acc. Chem. Res. 2004, 37, 497. (d) Shi, Y. Acc. Chem. Res. 2004, 37, 488. (d) Wang, Y.; Han, R. G.; Zhao, Y. L.; Yang, S.; Xu, P. F. Dixon, D. J. Angew. Chem., Int. Ed. 2009, 48, 9834.
[36] Wang, Y.; Yang, H.; Yu, J.; Miao, Z.; Chen, R. Adv. Synth. Catal. 2009, 351, 3057.
[37] Wang, Y.; Yu, J.; Miao, Z.; Chen, R. Org. Biomol. Chem. 2011, 9, 3050.
[38] Frings, M.; Thomé, I.; Bolm, C. Beilstein J. Org. Chem. 2012, 8, 1443.
[39] Ding, D.; Zhao, C.-G. Eur. J. Org. Chem. 2010, 2010, 3802.
[40] Xu, D.-Z.; Li, H.; Wang, Y. Tetrahedron 2012, 68, 7867.
[41] An, D.; Fan, Y.-S.; Gao, Y.; Zhu, Z.-Q.; Zheng, L.-Y.; Zhang, S.-Q. Eur. J. Org. Chem. 2014, 2014, 301.
/
| 〈 |
|
〉 |