

1-(1-乙基-1H-5-吲哚基)-2-苯基-1,2-二酮缩合成1,2,4-三嗪类化合物反应
Condensation of 1-(1-Ethyl-1H-indol-5-yl)-2-phenylethane- 1,2-dione to 1,2,4-Triazines
Received date: 2015-09-08
Revised date: 2015-10-06
Online published: 2015-10-13
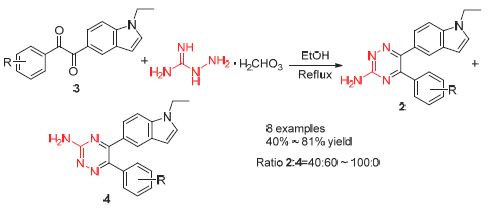
报道了一种1-(1-乙基-1H-5-吲哚基)-2-苯基-1,2-二酮与氨基胍碳酸氢盐缩合生成互为同分异构体的两种1,2,4-三嗪的反应, 并通过运用目标定向合成和核磁的方法, 研究了1,2-二酮苯环上不同取代基对反应产品比例的影响. 反应总收率为40%~81%, 且当不对称1,2-二酮苯环上无取代基时, 其生成的同分异构体6-(1-乙基-1H-5-吲哚基)-5-苯基- 3-氨基-1,2,4-三嗪(2a)和5-(1-乙基-1H-5-吲哚基)-6-苯基-3-氨基-1,2,4-三嗪(4a)比例为40:60; 当苯环上取代基为吸电子基时, 6-(1-乙基-1H-5-吲哚基)-5-(4-硝基苯基)-3-氨基-1,2,4-三嗪(2b)和5-(1-乙基-1H-5-吲哚基)-6-(4-硝基苯基)-3-氨基-1,2,4-三嗪(4b)比例为63:37; 当苯环上取代基为供电子基时, 绝大部分生成2系列构型结构.
刘剑功 , 黎晓龙 , 程旭 , 郝文梅 , 海俐 , 吴勇 . 1-(1-乙基-1H-5-吲哚基)-2-苯基-1,2-二酮缩合成1,2,4-三嗪类化合物反应[J]. 有机化学, 2016 , 36(2) : 370 -376 . DOI: 10.6023/cjoc201509010
This paper reports the selective condensation of aminoguanidine with 1-(1-ethyl-1H-indol-5-yl)-2-phenylethane- 1,2-dione to afford 1,2,4-triazines. The constituents and relative proportion of these condensation products are identified by the methods of target-oriented synthesis and nuclear magnetic resonance. And the influences on the relative proportion by different substituents are described. When the phenyl is substituted with the electron-withdrawing group, the ratio of the isomers 6-(1-ethyl-1H-indol-5-yl)-5-phenyl-1,2,4-triazin-3-amine/5-(1-ethyl-1H-indol-5-yl)-6-phenyl-1,2,4-triazin-3-amine [n(2a):n(4a)] is 40:60; when without the substituent, the ratio of the isomers 6-(1-ethyl-1H-indol-5-yl)-5-(4-nitrophenyl)- 1,2,4-triazin-3-amine/5-(1-ethyl-1H-indol-5-yl)-6-(4-nitrophenyl)-1,2,4-triazin-3-amine [n(2b):n(4b)] is 63:37; when substituted with the electron-donating group, the type of the major products was 2.

Key words: condensation; unsymmetrical 1,2-diketone; isomer; 1,2,4-triazine
[1] Lu, Z.-E.; Wan, J.; Chen, K. Q. Chin. J. Org. Chem. 1992, 12, 605 (in Chinese). (陆忠娥, 万军, 陈克潜, 有机化学, 1992, 12, 605.)
[2] Li, Y. J.; Shao, X.; Gao, L. X.; Jin, K.; Sheng, L.; Luo, T. C.; Yu, Y.; Li, J. Chin. J. Org. Chem. 2013, 33, 2178 (in Chinese). (李英俊, 邵昕, 高立信, 靳焜, 盛丽, 罗潼川, 于洋, 李佳, 有机化学, 2013, 33, 2178.)
[3] Li, Y. J.; Shi, X. L.; Gao, L. X.; Jin, K.; Sheng, L.; Wu, J. H.; Peng, L. N.; Li, J. Chin. J. Org. Chem. 2015, 35, 191 (in Chinese). (李英俊, 史相玲, 高立信, 靳焜, 盛丽, 吴疆红, 彭立娜, 李佳, 有机化学, 2015, 35, 191.)
[4] Eid, M. M.; Mansour, A. K. J. Heterocycl. Chem. 1988, 25, 1139.
[5] Rees, R. W. A.; Russell, P. B.; Foell, T. J.; Bright, R. E. J. Med. Chem. 1972, 15, 859.
[6] March, L. C.; Bajwa, G. S.; Lee, J.; Wasti, K.; Joullie, M. M. J. Med. Chem. 1976, 19, 845.
[7] Kim, J. W.; Lee, D. Y.; Park, C. M. ACS Med. Chem. Lett. 2012, 3, 678.
[8] Congreve, M.; Andrews, S. P.; Dore, A. S.; Hollenstein, K. J. Med. Chem. 2012, 55, 1898.
[9] Xie, F. C.; Zhao, H. B.; Li, D. W.; Chen, H.; Quan, H. T.; Shi, X. J.; Lou, L. G.; Hu, Y. H. J. Med. Chem. 2011, 54, 3200.
[10] Mallikarjuna, B. P.; Suresh, K. G. V.; Sastry, B. S.; Nagaraj; Manohara, K. P. J. Zhejiang Univ., Sci. B 2007, 8, 526.
[11] Cheng, X.; Li, X. L.; Wan, W. L.; Hao, W. M.; Hai, L.; Wu, Y. Chem. Res. Chin. Univ. 2015, 31, 53.
[12] Congreve, M.; Andrews, S. P.; Mason, J. S.; Richardson, C. M.; Brown, G. A. WO 2011095625, 2011 [Chem. Abstr. 2011, 155, 271296].
/
| 〈 |
|
〉 |