

CF3I催化合成中氟夺取途径的密度泛涵理论研究
收稿日期: 2015-09-20
修回日期: 2015-10-17
网络出版日期: 2015-10-26
Density Functional Theory Study on Fluorine Abstraction of the CF3I Catalytic Synthesis
Received date: 2015-09-20
Revised date: 2015-10-17
Online published: 2015-10-26
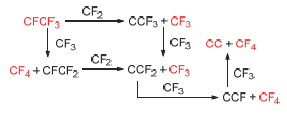
CF3I在制冷剂、刻蚀剂、发泡剂、灭火剂以及有机氟工业等领域具有广泛的应用. 在活性炭负载碱金属盐的催化下, 以CHF3和碘为原料, 可直接合成CF3I. CF3及CF4分别是该反应过程中重要的中间产物和副产物, 而积碳则导致催化剂失活的重要原因. 因此研究它们的产生机理具有重要意义. 对此本文采用密度泛涵理论(DFT)方法进行了研究. 结果表明: CF2及CF3可以对吸附于graphite(001)表面的CFCF3进行多步氟夺取, 最终得到CF3、CF4以及C2自由基. 以上氟夺取反应产生的C2自由基也导致了实验中的积碳现象. 上述反应机理与现有的实验数据相符.
胡应杰 , 潘仁明 , 王万军 . CF3I催化合成中氟夺取途径的密度泛涵理论研究[J]. 有机化学, 2015 , 35(12) : 2522 -2528 . DOI: 10.6023/cjoc201509022
CF3I has been widely used in the fields such as refrigerants, etching agents, foaming agents, fire extinguishing agents and organic fluorine industry. By reacting CHF3 with I2 in the presence of a catalyst, CHF3 can transform into CF3I. Aiming at this catalytic process, possible reaction pathways for the generation of CF3 (an important intermediate), CF4 (a main byproduct) and coke (an important reason for the catalyst deactivation) are investigated with quantum chemistry methods using density functional theory (DFT). The results show that CF2 and CF3 could abstract the fluorine from CFCF3 in multisteps over graphite(001) surface to afford CF3, CF4 and C2 radical respectively. It is also found that the coke deposition in experiments is due to the above fluorine abstraction. The suggested mechanism is in agreement with available experimental data and theoretical computations.

Key words: CF3I; synthesis; CFCF3; fluorine abstraction; activated carbon
[1] Levy, R. A.; Zaitsev, V. B.; Aryusook, K. J. Mater. Res. 1998, 13, 2643.
[2] Christophorou, L. G.; Olthoff, J. K. J. Phys. Chem. Ref. Data 2000, 29, 553.
[3] Li, Y.; Patten, K. O.; Youn, D. Atmos. Chem. Phys. 2006, 6, 4559.
[4] Hamins, A.; Borthwick, P. Combust. Flame 1998, 112, 161.
[5] Su, J. Z.; Kim, A. K.; Mawhinney, J. R. J. Fire Prot. Eng. 1996, 8, 45.
[6] Su, J. Z.; Kim A. K. Fire Technol. 2002, 38, 7.
[7] Vinegar, A.; Jepson, G. W.; Hammann, S. J. Am. Ind. Hyg. Assoc. J. 1999, 60, 403.
[8] Duan, Y. Y.; Zhu, M. S.; Shi, L. Fluid Phase Equilib. 1997, 131, 233.
[9] Petrik, V.; Cahard, D. Tetrahedron Lett. 2007, 48, 3327.
[10] Nagasaki, N.; Morikuni, Y.; Kawada, K.; Arai, S. Catal. Today 2004, 88, 121.
[11] Nagasaki, N.; Suzuki, N.; Nakano, S.; Kunihiro, N. US 5892136, 1999 [Chem. Abstr. 1998, 129, 29362].
[12] Yang, G. C.; Lei, S.; Pan, R. M.; Quan, H. D. J. Fluorine Chem. 2009, 130, 231.
[13] Yang, G. C.; Jia, X. Q.; Pan, R. M.; Quan, H. D. J. Mol. Catal. A: Chem. 2009, 309, 184.
[14] Yang, G. C.; Jia, X. Q.; Pan, R. M. J. Fluorine Chem. 2009, 130, 985.
[15] Hu, Y. J.; Wu, T. P.; Liu, W. Z.; Pan, R. M. J. Phys. Chem. A 2014, 118, 1918.
[16] Figueiredo, J.; Pereira, M.; Freitas, M.; Orfao, J. Carbon 1999, 37, 1379.
[17] McDougall, G. J. J. South. Afr. Inst. Min. Metall. 1991, 91, 109.
[18] Rodriguez-Reinoso, F. Carbon 1998, 36, 159.
[19] Delley, B. J. Chem. Phys. 2000, 113, 7756.
[20] Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865.
[21] Delley, B. J. Chem. Phys. 1990, 92, 508.
[22] Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. J. Chem. Phys. 1987, 86, 866.
[23] Bergner, A.; Dolg, M.; Küchle, W. Mol. Phys. 1993, 80, 1431.
[24] Halgren, T. A.; Lipscomb, W. N. Chem. Phys. Lett. 1977, 49, 225.
[25] Henkelman, G.; Jonsson, H. J. Chem. Phys. 2000, 113, 9978.
/
| 〈 |
|
〉 |