

新型二氢叶酸还原酶抑制剂的合成及生物活性测试
收稿日期: 2015-09-29
修回日期: 2015-10-30
网络出版日期: 2015-11-06
基金资助
国家科技重大新药创制专项(No. 2010ZX09401-404)资助项目.
Synthesis and Bioactivity of Novel Dihydrofolate Reductase Inhibitor
Received date: 2015-09-29
Revised date: 2015-10-30
Online published: 2015-11-06
Supported by
Project supported by the National Significant and Special Project of New Created Drugs (No. 2010ZX09401-404).
以氯乙腈和2-噻吩甲醛为原料, 通过接合经缩合、环合、还原、硝化等步骤合成的两个关键中间体1c和2d, 设计合成了两个新型二氢叶酸还原酶抑制剂的衍生物3c和4b, 其结构经1H NMR、13C NMR和MS方法验证. 采用噻唑蓝法对目标化合物进行了抗肿瘤活性测试, 结果表明3c对筛选的5种肿瘤细胞株的抑制活性均比阳性对照药洛美曲索、甲氨蝶呤和培美曲塞强, 4b对Hep-G2的抑制活性比阳性对照药洛美曲索、甲氨蝶呤和培美曲塞强, 而且3c和4b对正常细胞株——人脐带内皮血管平滑肌细胞的抑制活性明显比甲氨蝶呤和培美曲塞弱.
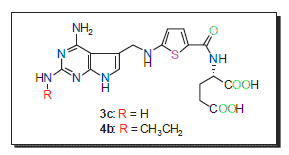
关键词: 二氢叶酸还原酶抑制剂; 抗肿瘤活性; 合成
张袁魁 , 陈简 , 詹晓平 , 徐赟 , 刘增路 , 毛振民 . 新型二氢叶酸还原酶抑制剂的合成及生物活性测试[J]. 有机化学, 2016 , 36(3) : 664 -669 . DOI: 10.6023/cjoc201509042
In order to obtain antitumor candidates with potent activity, two novel analogues of dihydrofolate reductase (DHFR) inhibitors 3c and 4b were designed and synthesized. The synthetic routes were as follows: 1c was synthesized using chloroacetonitrile, carboxylate and 2,4,6-triamino-pyrimidine as raw materials by condensation, cyclization and reduction. 2d was synthesized using 2-thiophene formaldehyde and glutamate as raw materials by nitrification, oxidation, condensation and reduction. Then target product 3c was synthesized using 1c and 2d as raw materials by reductive amination and hydrolysis. Target product 4b was synthesized using 1c and 2d as raw materials by 2 steps of reductive amination and hydrolysis. The structures of target products were confirmed by the way of 1H NMR, 13C NMR and MS. The in vitro antitumor activities of target products were evaluated by MTT method. The results showed that 3c exhibited stronger antitumor activity against screened five tumor cell lines (CCRF-CEM, L1210, A549, SGC-7901 and Hep-G2) than the positive control lometrexol, methotrexate and pemetrexed. 3c exhibited stronger antitumor activity against Hep-G2 than the positive control lometrexol, methotrexate and pemetrexed. In addition, 3c and 4b showed much weaker antiproliferative activity toward normal cell SMC than the positive control methotrexate and pemetrexed. This research could provide a theoretical basis for the development of new antitumor drugs.

Key words: DHFR inhibitor; antitumor bioactivity; synthesis
[1] Wang, J.; Xu, Q.-L.; Li, J.-M.; Zhang, E.-L.; Hu, M.-H.; Ye, W.-F.; Huang, W.-J. Chin. J. Org. Chem. 2014, 34, 2040 (in Chinese). (王杰, 许勤龙, 李家明, 张恩立, 胡敏华, 叶文峰, 黄伟军, 有机化学, 2014, 34, 2040.)
[2] Cao, S.-L.; Guo, Y.-W.; Wang, X.-B. Chin. New Drugs J. 2007, 16, 747 (in Chinese). (曹胜利, 郭燕文, 王先波, 中国新药杂志, 16, 747.)
[3] Taylor, E. C.; Patel, H. H. Tetrahedron 1992, 48(37), 8089.
[4] Adjei, A. A. Ann. Oncol. 2000, 11(10), 1335.
[5] Emon, J. H.; Grindey, G. B. Cancer Res. 1978 38(9), 2905.
[6] Norris, R. E.; Adamson, P. C. Cancer Chemother. Pharmacol. 2010, 65(6), 1125.
[7] (a) Mendelsohn, L.-G.; Shih, C.; Schultz, R.-M.; Worzalla, J.-F. Invest. New Drugs 1996 14(3), 287.
(b) Taylor, E.-C.; Wong, G.-S.-K. J. Org. Chem. 1989 54(15), 3618.
[8] (a) Taylor, E. C.; Chaudhari, R.; Lee, K. Invest. New. Drugs 1996, 14(3), 281.
(b) Takimoto, C. H. Oncologist 1996, 1(1~2), 68.
(c) Borrell, J. I.; Teixidó, J.; Martínez-Teipel, B.; Matallana, J. L.; Copete, M. T.; Llimargas, A.; García, E. J. Med. Chem. 1998, 41(18), 3539.
[9] (a) Migawa, M.-T.; Hinkley, J.-M.; Hoops, G.-C. Synth. Commun. 1996, 26(17), 3317.
(b) Klepper, F.; Polborn, K.; Carecell, T. Helv. Chim. Acta 2005, 88(10), 2610.
[10] Gangjee, A.; Mavandadi, F.; Queener, S.-F. J. Med. Chem. 1995, 38(12), 2158.
[11] Gangjee, A.; Mavandadi, F.; Queener, S.-F. J. Med. Chem. 1997, 40(7), 1173.
[12] Alberto, B.; Adamo, F. US 4767758 [Chem. Abstr. 1987, 99, 20162].
[13] (a) Fu, B.; Li, N.; Liang, X.-M.; Dong, Y.-H.; Wang, D.-Q. Chin. J. Org. Chem. 2007, 27(1), 1 (in Chinese). (傅滨, 李楠, 梁晓梅, 董燕红, 王道全, 有机化学, 2007, 27(1), 1.)
(b) Sato, S.; Sakamoto, T.; Miyazawa, E.; Kikugawa, Y. Tetrahedron 2004, 60, 7899.
(c) Jung, Y.-J.; Bae, J.-W.; Park, E.-S.; Chang, Y.-M.; Yoon, C.-M. Tetrahedron 2003, 59, 10331
[14] Taylor, E.-C.; Hamby, J.-M.; Shih, C. J. Med. Chem. 1989, 32(7), 1517.
[15] Yu, X.-H.; Mao, Q.-H.; Zhang, W.-D. J. East China Univ. Sci. Technol., Nat. Sci. Ed. 2005, 31(2), 184 (in Chinese). (虞心红, 毛庆华, 张卫东等, 华东理工大学学报(自然科学版), 2005, 31(2), 184.)
[16] Hodson, S.-J.; Bigham, E.-C.; Duch, D.-S.; Smith, G.-K.; Ferone, R. J. Med. Chem. 1994, 37(13), 2112.
[17] Piper, J.-R.; DeGraw, J.-I.; Colwell, W.-T.; Johnson, C.-A.; Smith, R.-L.; Waud, W.-R.; Sirotnak, F.-M. J. Med. Chem. 1997, 40(3), 377.
/
| 〈 |
|
〉 |