

新型3-取代苯甲酰基-4-取代噻吩基吡咯类化合物的合成及抗肿瘤活性研究
收稿日期: 2015-10-14
修回日期: 2015-11-06
网络出版日期: 2015-11-20
基金资助
国家科技重大新药创制专项(No. 2010ZX09401404-004)资助项目.
Synthesis and Anti-tumor Activity of Novel 3-Substituted-benzoyl-4-substituted-thienyl-pyrroles
Received date: 2015-10-14
Revised date: 2015-11-06
Online published: 2015-11-20
Supported by
Project supported by the National Science and Technology Major Special Drug Discovery (No. 2010ZX09401404-004).
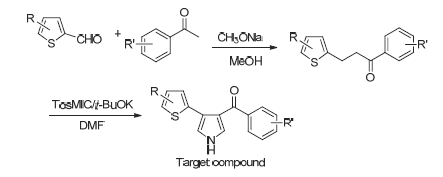
以取代苯乙酮和取代噻吩-2-甲醛为原料, 经过Aldol缩合和Van Leusen吡咯合成法合成了29个未见文献报道的3-取代苯甲酰基-4-取代噻吩基吡咯类化合物2a~5c. 以噻唑蓝(MTT)法测定目标化合物对人结肠癌细胞 (HCT-116)、人胃腺癌细胞(SGC-7901)、人宫颈癌细胞(Hela)和H人脐静脉血管内皮细胞(UVEC)的细胞增殖抑制活性, 结果显示化合物2c, 2i, 3i, 3j, 4a~4f和5c对HCT-116细胞有较强(IC50≤20 μmol·L-1)或中等(20 μmol·L-150≤50 μmol·L-1)增殖抑制作用; 化合物2j对Hela细胞有较强增值抑制作用, 其IC50值为4.3 μmol·L-1, 化合物2i, 3j对Hela细胞有中等增殖抑制作用; 化合物3j对SGC-7901细胞有较强增殖抑制作用, 化合物2i对SGC-7901细胞有中等增殖抑制作用. 几乎所有化合物对HUVEC细胞无显著增殖抑制作用, 具有高选择性.
陈简 , 张袁魁 , 詹晓平 , 刘增路 , 毛振民 . 新型3-取代苯甲酰基-4-取代噻吩基吡咯类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2016 , 36(3) : 572 -579 . DOI: 10.6023/cjoc201510016
29 novel 3-substituted-benzoyl-4-substituted-thienyl-pyrrole compounds 2a~5c were synthesized via aldol condensation and Van Leusen pyrrole reaction using substituted acetophenone and substituted 2-thenaldehyde as raw materials, and the cell proliferation inhibition efficacy of 2a~5c against human colon cancer (HCT-116), human gastric adenocarcinoma (SGC-7901), human cervical carcinoma (Hela) and human umbilical vein endothelial (HUVEC) cell lines were estimated. Then MTT assay was used to evaluate the anti-proliferative activity. The result indicated that some target compounds exhibited strong (IC50≤20 μmol·L-1) or moderate (20 μmol·L-150≤50 μmol·L-1) proliferation inhibition efficacy against tumor cells, meanwhile didn't have significate inhibition effect on HUVEC. Compounds 2c, 2i, 3i, 3j, 4a~4f and 5c showed strong or moderate inhibition efficacy against HCT-116. The IC50 value of 2j was 4.3 μmol·L-1 against Hela, and compounds 2i and 3j exhibited moderate inhibition efficacy against Hela. The IC50 value of 3j was 10.5 μmol·L-1 against SGC-7901, and compounds 2i indicated moderate inhibition efficacy against SGC-7901. Compounds 2i and 3j showed broad anti-proliferative activity against all selected tumor cells.

Key words: pyrrole; thiophene; synthesis; MTT assay; anti-tumor activity
[1] Nordmann, J.; Müller, T. J. J. Org. Biomol. Chem. 2013, 11, 6556.
[2] Hopp, D. C.; Rhea, J.; Jacobsen, D.; Romari, K.; Smith, C.; Rabenstein, J.; Irigoyen, M.; Clarke, M.; Francis, L.; Luche, M.; Carr, G. J.; Mocek, U. J. Nat. Prod. 2009, 72, 276.
[3] Pham, V. C.; Shin, J.-S.; Choi, M. J.; Kim, T. W.; Lee, K.; Kim, K. J.; Huh, G.; Kim, J.; Choo, D. J.; Lee, K.-T.; Lee, J. Y. Bull. Korean Chem. 2012, 33, 721.
[4] Bellina, F.; Rossi, R. Tetrahedron 2006, 62, 7213
[5] Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P. N. Engl. J. Med. 2011, 364, 501.
[6] Gupton, J. T. Top Heterocycl. Chem. 2006, 2, 53.
[7] Lan, L.; Zhan, X.-P.; Qin, W.-X.; Liu, Z.-L.; Mao, Z.-M. Heterocycles 2014, 89, 375.
[8] Lan, L.; Qin, W.-X.; Zhan, X.-P.; Liu, Z.-L.; Mao, Z.-M. Anti-Cancer Agents Med. Chem. 2014, 14, 994.
[9] Yin, C.-Q.; Zhan, X.-P.; Lan, L.; Qin, W.-X.; Liu, Z.-L.; Mao, Z.-M. Prog. Modern Biomed. 2014, 14, 5419 (in Chinese). (殷昌青, 詹晓平, 兰岚, 覃维曦, 刘增路, 毛振民, 现代生物医学进展, 2014, 14, 5419.)
[10] Barbarella, G.; Melucci, M.; Sotgiu, G. Adv. Mater. 2005, 17, 1581.
[11] Lu, Y.; Yuan, S.-B. Fine Spec. Chem. 2002, 22, 5 (in Chinese). (陆咏, 袁少波, 精细与专用化学品, 2002, 22, 5.)
[12] Huang, Y.-H.; Li, D.-P.; Chen, L.-S. J. Leukemia Lymphoma 2003, 12, 271 (in Chinese). (黄一虹, 李德鹏, 陈令松, 白血病·淋巴瘤, 2003, 12, 271.)
[13] Sharma, R.; Kumar, K.; Chouhan, M.; Grover, V.; Nair, V. A. RSC Adv. 2013, 3, 14521.
[14] van Leusen, A. M.; Siderius, H.; Hoogenboom, B. E.; van Leusen, D. Tetrahedron Lett. 1972, 52, 5337.
[15] Heo, D. S.; Park, J.-G.; Hata, K.; Day, R.; Herberman, R. B.; Whiteside, T. L. Cancer Res. 1990, 50, 3681.
[16] Dannhardt, G.; Kiefer, W.; Krämer, G.; Maehrlein, S.; Nowe, U.; Fiebich, B. Eur. J. Med. Chem. 2000, 35, 499.
/
| 〈 |
|
〉 |