

天然产物BrosimacutinsH和I的对映选择性全合成
收稿日期: 2015-08-24
修回日期: 2015-11-06
网络出版日期: 2015-11-26
基金资助
国家自然科学基金(Nos. 21362025)、教育部"新世纪优秀人才支持计划"(No. NCET-09-0860)资助项目.
First Enantioselective Synthesis of Brosimacutins H and I
Received date: 2015-08-24
Revised date: 2015-11-06
Online published: 2015-11-26
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21362025) and the Program for New Century Excellent Talents in University (No. NCET-09-0860) .
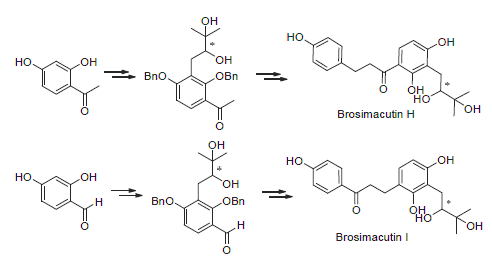
Brosimacutins H和I是从巴西的Brosimum acutifolium Huber树皮中分离出的两个具有相似结构的黄酮类化合物. 此树皮被巴西当地居民作为抗发炎和抗风湿的药物, 并且这两种化合物具有一定的细胞活性. 以廉价的羟苯乙酮和羟苯甲醛为原料完成了黄酮化合物Brosimacutins H和I的对映选择性合成. 所有新化合物的结构都经过NMR, HRMS确认.
关键词: brosimacutin; 异戊烯基黄酮; 查尔酮; 二氢查尔酮; 全合成
叶子平 , 杨金会 , 冯尧 , 马涛 , 牛明杰 . 天然产物BrosimacutinsH和I的对映选择性全合成[J]. 有机化学, 2016 , 36(3) : 547 -554 . DOI: 10.6023/cjoc201508021
Brosimacutins H and I, isolated from the bark of brosimum acutifolium huber, are flavanoid compounds with similar structures. The bark of this plant is used in Brazilian folk medicine as an anti-inflammatory and anti-rheumatic agent, and cellular activities were reported for these two compounds. Herein the first enantio-selective synthesis of brosimacutins H and I from cheap starting material hydroxyl-acetophenone and hydroxyl benzene formaldehyde was reported. All new compounds in this study were confirmed by NMR and HRMS.

Key words: brosimacutin; prenylated flavonoids; chalcone; dihydrochalcones; total synthesis
[1] Andersen, Ø. M.; Markham, K. R. Flavonoids, Chemistry, Biochemistry and Applications, Taylor & Francis, London, 2006.
[2] Daskiewicz, J. B.; Depeint, F.; Viornery, L.; Bayet, C.; Comte-Sarrazin, G.; Comte, G.; Gee, J. M.; Johnson, I. T.; Ndjoko, K. K.; Hostettmann, K.; Barron, D. J. Med. Chem. 2005, 48, 2790.
[3] Meragelman, T. L.; Tucker, K. D.; McClord, T. G.; Cardel-lina, J. H.; Shoemker, R. H. J. Nat. Prod. 2005, 68, 1790.
[4] Na, M.; Jang, J.; Njamen, D.; Mbafor, J. T.; Fomum, Z. T.; Kim, B. Y.; Oh, W. K.; Ahn, J. S. J. Nat. Prod. 2006, 69, 1572.
[5] Prestos, C.; Boziaris, I. S.; Nychas, J. E. Food Chem. 2005, 93, 1998.
[6] Yoon, H.; Kim, T. W.; Shin, S. Y.; Park, M. J.; Yong, Y.; Kim, D. W.; Islam, T.; Lee, Y. H.; Jung, K. Y.; Lim, Y. Biol. Med. Chem. Lett. 2013, 23, 232.
[7] Tamotsu, N.; Taichi, O.; Takeshi, K. Chem. Pharm. Bull. 1989, 37, 1392.
[8] Barron, D.; Ibraham, R. K. Phytochemistry 1996, 43, 921.
[9] Takashima, J.; Ohsaki, A. J. Nat. Prod. 2002, 65, 1483.
[10] Takashima, J.; Ohsaki, A. Planta Med. 2005, 71, 654.
[11] Feng, Y.; Yang, J. H.; Ma, X. Q.; Cheng, B. B.; Xie, Y. M. Chin. J. Org. Chem. 2014, 34, 2015 (in Chinese). (冯尧, 杨金会, 马晓琴, 陈兵兵, 谢一民, 有机化学, 2014, 34, 2015.)
[12] Zuo, W. B.; Yang, J. H.; Li, H. J.; Guo, D. D.; Luo, J. S.; Huang, W. Q. Chin. J. Org. Chem. 2012, 32, 2276 (in Chinese). (左武标, 杨金会, 李红俊, 郭冬冬, 落俊山, 黄文倩, 有机化学, 2012, 32, 2276.)
[13] Yang, J. H.; Huang, W. Q.; Luo, J. S.; Guo, D. D.; Zhang, Y. H.; Li, H. J. Chin. Chem. Lett. 2012, 23, 127.
[14] Yang, J. H.; Chen, B. B.; Xie, Y. M.; Feng, Y.; Ma, X. Q. Chin. Chem. Lett. 2013, 24, 1027.
[15] Yang, J. H.; Xie, Y. M.; Feng, S. B.; Zuo, W. B.; Cheng, B. B.; Ma, X. Q.; Feng, Y. Chin. J. Org. Chem. 2012, 32, 2159 (in Chinese). (杨金会, 谢一民, 冯尚彪, 左武标, 陈兵兵, 马晓琴, 冯尧, 有机化学, 2012, 32, 2159.)
[16] Ma, X. Q.; Yang, J. H.; Feng, Y.; Cheng, B. B.; Xie, Y. M. Chin. J. Org. Chem. 2014, 34, 2001 (in Chinese). (马晓琴, 杨金会, 冯尧, 陈兵兵, 谢一民, 有机化学, 2014, 34, 2001.)
[17] Fristrup, P.; Jensen, G. H.; Andersen, M. L. N.; Tanner, D.; Norrby, P. O. J. Organomet. Chem. 2006, 2182.
/
| 〈 |
|
〉 |