

水溶性吡啶萘酰亚胺-聚酰胺-胺荧光树形分子的合成、聚集诱导荧光增强及细胞成像
收稿日期: 2015-09-30
修回日期: 2015-11-06
网络出版日期: 2015-11-26
基金资助
国家自然科学基金(No. 61178057)资助项目.
Synthesis, Aggregation-Induced Emission Enhancement and Cells Imaging of Water-Soluble Pyridine Naphthalimide-Polyamidoamine Dendrimer
Received date: 2015-09-30
Revised date: 2015-11-06
Online published: 2015-11-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 61178057).
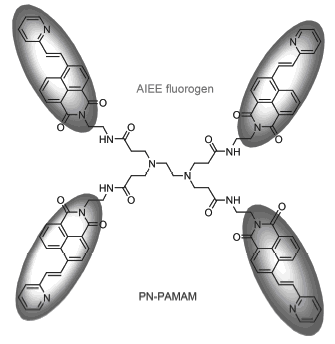
将具有聚集诱导荧光的4-(2-吡啶乙烯基)-1,8-萘酰亚胺单元通过酰胺化反应连接到聚酰胺-胺(PAMAM)树形分子上, 制备了一种水溶性吡啶萘酰亚胺-聚酰胺-胺荧光树形分子PN-PAMAM. 结构经核磁共振氢谱、核磁共振碳谱、红外及高分辨质谱表征. 吡啶萘酰亚胺-聚酰胺-胺树形分子PN-PAMAM具有明显的聚集诱导荧光增强(AIEE)特性. 在固体态时的最大荧光发射波长为532 nm, 紫外灯下发出黄绿色荧光. 在纯水中的最大荧光发射波长为494 nm, 荧光量子产率为4.42%. 在水含量为60%的水/四氢呋喃混合溶液中, 其荧光强度达到最大, 荧光发射波长为485 nm, 荧光量子产率增大到17.54%. 制备了一种负载聚集诱导荧光染料PN-PAMAM的二氧化硅纳米粒子PN-PAMAM/SiO2, 测得该纳米粒子的荧光发射波长为473 nm, 粒径约为40 nm. 测定了水溶液中树形分子PN-PAMAM与乳腺癌细胞MCF-7共同孵化后的共聚焦荧光成像, 得到清晰的蓝场荧光照片. 研究表明, 吡啶萘酰亚胺-聚酰胺-胺树形分子PN-PAMAM是一种水溶性荧光分子, 具有明显的聚集诱导荧光增强特性, 可广泛应用于肿瘤定位、生物追踪及纳米材料等重要领域.
关键词: 吡啶萘酰亚胺-聚酰胺-胺树形分子; 水溶性荧光分子; 聚集诱导荧光增强; 纳米粒子; 细胞成像
牛艳芳 , 钱鹰 , 胡秀东 . 水溶性吡啶萘酰亚胺-聚酰胺-胺荧光树形分子的合成、聚集诱导荧光增强及细胞成像[J]. 有机化学, 2016 , 36(3) : 555 -561 . DOI: 10.6023/cjoc201509045
A water-soluble pyridine naphthalimide-polyamidoamine dendrimer (PN-PAMAM) was synthesized by the amidation reaction of 4-(2-vinylpyridine)-1,8-naphthalimide unit and the polyamidoamine (PAMAM). The structure was characterized by 1H NMR, 13C NMR, IR and HRMS techniques. PN-PAMAM displayed obvious aggregation-induced emission enhancement (AIEE) behavior. The experimental results demonstrated that dendrimer PN-PAMAM in solid state emitted a yellow-green fluorescence and the maximum emission wavelength was 532 nm. In aqueous solution the maximum emission wavelength was 494 nm and the fluorescence quantum yield was 4.42%. The fluorescence intensity reached the maximum at 60% water volume fraction in H2O/THF mixed solution. The fluorescence emission wavelength was 485 nm and the fluorescence quantum yield was increased to 17.54%. The photophysical properties of the silica nanoparticles loaded AIEE dye PN-PAMAM were examined. The fluorescence emission wavelength was 473 nm and the particle diameter was about 40 nm. In addition, dendrimer PN-PAMAM were applied for breast cancer MCF-7 cells imaging, getting clear blue-field photograph. In summary, the pyridine naphthalimide-PAMAM dendrimer is a water-soluble fluorescent dye and displays AIEE behavior. PN-PAMAM can be widely used in areas of tumor localization, bio-tracking and nano-materials.

[1] Luo, J.-D.; Xie, Z.-L.; Lam, J. W. Y.; Cheng, L; Chen, H.-Y.; Qiu, C.-F.; Kwok, H. S.; Zhan, X.-W.; Liu, Y.-Q.; Zhu, D.-B.; Tang, B.-Z. Chem. Commun. 2001, 18, 1740.
[2] Hong, Y.-N.; Lam, J. W. Y.; Tang, B.-Z. Chem. Commun. 2009, 29, 4332.
[3] Liu, J.-Z.; Lam, J. W. Y.; Tang, B.-Z. J. Inorg. Organomet. Polym. 2009, 19, 249.
[4] Chen, J.-W.; Law, C. C. W.; Lam, J. W. Y.; Dong, Y.-P.; Lo, S. M. F.; Williams, I. D.; Zhu, D.-B.; Tang, B.-Z. Chem. Mater. 2003, 15, 1535.
[5] Hong, Y.-N.; Lam, J. W. Y.; Tang, B.-Z. Chem. Soc. Rev. 2011, 40, 5361.
[6] Zhao, Z.-J.; Lam, J. W. Y.; Tang, B.-Z. J. Mater. Chem. 2012, 22, 23726.
[7] Leung, C. W. T.; Hong, Y.-N.; Chen, S.-J.; Zhao, E.-G.; Lam, J. W. Y.; Tang, B.-Z. J. Am. Chem. Soc. 2013, 135, 62.
[8] Hu, R.-R.; Lam, J. W. Y.; Liu, Y.; Zhang X.-A.; Tang, B.-Z. Chem. Eur. J. 2013, 19, 5617.
[9] Yuan, W.-Z.; Gong, Y.-Y.; Chen, S.-M.; Shen, X.-Y.; Lam, J. W. Y.; Lu, P.; Lu, Y.-W.; Wang, Z.-M.; Hu, R.-R.; Xie, N,; R.-R.; Kwok, H. S. Zhang, Y.-M.; Sun, J.-Z.; Tang, B.-Z. Chem. Mater. 2012, 24, 1518.
[10] Zhu, L.; Younes, A.; Yuan, Z.; Clark, R. J. J. Photochem. Photobiol. Chem. 2015, 311, 1.
[11] Zhu, L.; Yuan, Z.; Simmons, J. T.; Sreenath, K. RSC Adv. 2014, 4, 20398.
[12] Zhang, G.-F.; Aldred, M. P.; Gong, W.-L.; Li, C.; Zhu. M.-Q. Chem. Commun. 2012, 48, 7711.
[13] Sun, Y.; Liang, X.-H.; Wei, S.; Fan, J.; Yang, X.-H. Spectrochim. Acta, Part A. 2012, 97, 352.
[14] Sun, Y.; Liang, X.-H.; Fan, J.; Han, Q. J. Lumin. 2013, 141, 93.
[15] Ottaviani, M. F.; Cossu, E.; Turro, N. J.; Tomalia, D. A. J. Am. Chem. Soc. 1995, 117, 4387.
[16] Tomalia, D. A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Polym. J. 1985, 17, 117.
[17] Zhang, Y.; Xu, M.-Y.; Jiang, T.-K.; Huang, W.-Z.; Wu, J.-Y. Chin. Chem. Lett. 2014, 25, 815.
[18] Ji, Y.; Qian, Y. RSC Adv. 2014, 4, 58788.
[19] Scholl, M.; Kadlecova, Z.; Klok, H. A. Prog. Polym. Sci. 2009, 34, 24.
[20] Zeng, Y.; Li, Y.-Y.; Yuan, Z.; Li, Y. Acta Chim. Sinica 2009, 67, 2714 (in Chinese). (曾毅, 李迎迎, 袁钊, 李嫕, 化学学报, 2009, 67, 2714.)
[21] Yuan, Z.; Liang, F. Cur. Org. Chem. 2014, 18, 2016.
[22] Ji, Y.; Qian, Y. RSC Adv. 2014, 4, 25510.
[23] Grabchev, I.; Qian, X.-H.; Bojinov, V.; Xiao, Y.; Zhang, W. Polymer 2002, 43, 5731.
[24] Grabchev, I.; Chovelon, J. M.; Qian, X.-H. New J. Chem. 2003, 27, 337.
[25] Grabchev, I.; Betcheva, R.; Bojinov, V.; Staneva, D. Eur. Polym. J. 2004, 40, 1249.
[26] Refat, M. S.; Teleb, S. M.; Grabchev, I. Spectrochim. Acta, Part A. 2005, 61, 205.
[27] Sali, S.; Grabchev, I.; Chovelon, J. M.; Ivanova, G. Spectrochim. Acta, Part A 2006, 65, 591.
[28] Grabchev, I.; Chovelon, J. M.; Petkov, C. Spectrochim. Acta, Part A 2008, 69, 100.
[29] Grabchev, I.; Bosch, P.; Staneva, D. J. Photochem. Photobiol. A 2011, 222, 288.
[30] Staneva, D.; Bosch, P.; Asiri, A. M.; Taib, L. A.; Grabchev, I. Dyes Pigm. 2014, 105, 114.
[31] Yordanova, S.; Grabchev, I.; Stoyanov, S.; Petkov, I. J. Photochem. Photobiol. A: Chem. 2014, 283, 1.
[32] Luo, X.-Y.; Qian, Y. Chin. J. Org. Chem. 2013, 33, 2423 (in Chinese). (罗晓燕, 钱鹰, 有机化学, 2013, 33, 2423.)
[33] Sun, J.-F.; Qian, Y. Chin. J. Org. Chem. 2015, 35, 1104 (in Chinese). (孙京府, 钱鹰, 有机化学, 2015, 35, 1104.)
[34] Guan, C.-F.; Qian, Y. Chin. J. Org. Chem. 2014, 34, 537 (in Chinese). (管成飞, 钱鹰, 有机化学, 2014, 34, 537.)
[35] Luo, M.-L.; Qian, Y. Chin. J. Org. Chem. 2012, 32, 1958 (in Chinese). (罗蔓利, 钱鹰, 有机化学, 2012, 32, 1958.)
[36] Chen, H.-R.; Qian, Y. Dyes Pigm. 2015, 112, 317.
[37] Tao, Z.-Q.; Qian, Y. Chin. J. Org. Chem. 2014, 34, 2354 (in Chinese). (陶在琴, 钱鹰, 有机化学, 2014, 34, 2354.)
[38] Gelamo, E. L., Silva, C. H. T. P., Imasato, H., Tabak, M. Biochim. Biophys. Acta 2002, 1594, 84.
[39] Zhang, Y.; Qi, Z.-D.; Zheng, D.; Li, C.-H.; Liu, Y. Biol. Trace Elem. Res. 2009, 130, 172.
[40] Zhang, C.-H.; Zang, S.-L.; Geng, B.; Tie, M.; Wu, L.-Y.; Su, X.; Feng, C. Chin. J. Anal. Sci. 2005, 21, 179 (in Chinese). (张朝红, 臧树良, 耿兵, 铁梅, 吴林友, 苏欣, 冯冲, 分析化学, 2005, 21, 179.)
[41] Zheng, S.; Yuan, Z.; Zeng, Y.; Li, Y.-Y.; Li, Y. Acta Phys. Chim. Sin. 2008, 24, 1785 (in Chinese). (郑少君, 袁钊, 曾毅, 李迎迎, 李嫕, 物理化学学报, 2008, 24, 1785.)
[42] Jin, Y.-C.; Qian, Y. New J. Chem, 2015, 39, 2872.
[43] Gu, P.-Y.; Lu, C.-J.; Hu, Z.-J.; Li, N.-J.; Zhao, T.-T.; Xu, Q.-F.; Xu, Q.-H.; Zhang, J.-D.; Lu, J.-M. J. Mater. Chem. C 2013, 1, 2599.
[44] Demasa, J. N.; Crosby, G. A. J. Phys. Chem. 1971, 76, 991.
/
| 〈 |
|
〉 |