

琼脂糖水凝胶包埋柠檬酸三钠催化多组分反应合成苯并吡喃及吡喃并吡唑衍生物
收稿日期: 2015-10-11
修回日期: 2015-11-25
网络出版日期: 2015-12-07
基金资助
国家自然科学基金(Nos.21372099,21072077)和广东省自然科学基金(No.10151063201000051,8151063201000016)资助项目.
Agarose Hydrogel Entrapped Trisodium Citrate Catalyzed Multicomponent Reactions for the Synthesis of Benzopyran and Pyranopyrazole Derivatives
Received date: 2015-10-11
Revised date: 2015-11-25
Online published: 2015-12-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21372099, 21072077), the Guangdong Natural Science Foundation (Nos. 10151063201000051, 8151063201000016).
报道了琼脂糖水凝胶包埋柠檬酸三钠催化剂(Gel-Citrate)高效催化多组分反应合成一系列苯并吡喃衍生物和吡喃并[2,3-c]吡唑衍生物的方法.该方法具有催化剂制备简单、对环境友好、操作简单方便、反应条件温和、产率优良等优点.且催化剂可回收重复使用6次,产率没有明显降低.
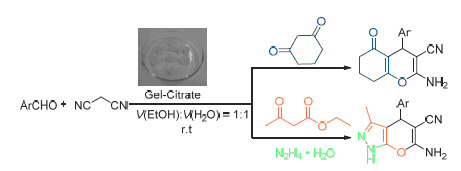
关键词: 琼脂糖; 水凝胶包埋催化剂; 苯并吡喃; 吡喃并[2,3-c]吡唑; 多组分反应
李志鹏 , 陈斐然 , 王东阳 , 黄杏甜 , 李毅群 . 琼脂糖水凝胶包埋柠檬酸三钠催化多组分反应合成苯并吡喃及吡喃并吡唑衍生物[J]. 有机化学, 2016 , 36(4) : 838 -843 . DOI: 10.6023/cjoc201510010
An efficient method for the synthesis of benzopyran derivatives and pyrano[2,3-c]pyrazole derivatives via multicomponent reaction in the presence of agarose hydrogel entrapped trisodium citrate (gel-citrate) as catalyst is developed. The protocol presented here has the merits of easy preparation and handling of catalyst, environmentally benign, simple operation and convenient workup, and excellent yields. Moreover, the catalyst could be recovered and reused at least 6 cycles without apparently losing its activities.

[1] Strecker, A. Ann. 1850, 75, 27.
[2] Hantzsch, A. Ber. Dtsch. Chem. Ges. 1881, 14, 1637.
[3] Mannich, C.; Krösche, W. Arch. Pharm. 1912, 250, 647.
[4] Ugi, I. Angew. Chem., Int. Ed. Engl. 1962, 1, 8.
[5] (a) Ruijter, E.; Scheffelaar, R.; Orru, R. V. Angew. Chem., Int. Ed. Engl. 2011, 50, 6234.
(b) Wang, J.; Shen, Q.; Li, P.; Peng, Y.; Song, G. Org. Biomol. Chem. 2014, 12, 5597.
(c) Banfi, L.; Basso, A.; Moni, L.; Riva, R. Eur. J. Org. Chem. 2014, 2014, 2005.
(d) Bharti, R.; Parvin, T. RSC Adv. 2015, 5, 66833.
(e) Nikbakht, A.; Ramezanpour, S.; Balalaie, S.; Rominger, F. Tetrahedron 2015, 71, 6790.
(f) Liu, B.; Wei, E.; Lin, S.; Zhao, B.; Liang, F. Chem. Commun. 2014, 50, 6995.
[6] (a) Wang, Q. F.; Song, X. K.; Yan, C. G. Prog. Chem. 2009, 21, 997 (in Chinese). (王琦芳, 宋肖锴, 颜朝国, 化学进展, 2009, 21, 997.)
(b) Guo, H. Y.; Tian, J. J. Chin. J. Org. Chem. 2011, 31, 1752 (in Chinese). (郭红云, 田金金, 有机化学, 2011, 31, 1752.)
(c) Han, Y.; Sun, J.; Sun, Y.; Gao, H.; Yan, C. G. Chin. J. Org. Chem. 2012, 32, 1577 (in Chinese). (韩莹, 孙晶, 孙岩, 高红, 颜朝国, 有机化学, 2012, 32, 1577.)
(d) Xiao, L. W.; Peng, X. X.; Zhou, Q. X.; Kou, W.; Shi, Y. R. Chin. J. Org. Chem. 2015, 35, 1204 (in Chinese). (肖立伟, 彭晓霞, 周秋香, 寇伟, 时亚茹, 有机化学, 2015, 35, 1204.)
(e) Tian, J. J.; Guo, H. Y. Chin. J. Org. Chem. 2011, 31, 2009 (in Chinese). (田金金, 郭红云, 有机化学, 2011, 31, 2009.)
[7] (a) Bonsignore, L.; Loy, G.; Secci, D.; Calignano, A. Eur. J. Med. Chem. 1993, 28, 517.
(b) Zhang, G.; Zhang, Y. H.; Yan, J. X.; Chen, R.; Wang, S. L.; Ma, Y. X.; Wang, R. J. Org. Chem. 2012, 77, 878.
(c) Kuo, S. C.; Huang, L. J.; Nakamura, H. J. Med. Chem. 1984, 27, 539.
(d) Wang, J.-L.; Liu, D.; Zhang, Z.-J.; Shan, S.; Han, X.; Srinivasula, S. M.; Croce, C. M.; Alnemri, E. S.; Huang, Z. P. Natl. Acad. Sci. 2000, 97, 7124.
(e) Abdelrazek, F. M.; Metz, P.; Metwally, N. H.; El-Mahrouky, S. F. Arch. Pharm. 2006, 339, 456.
[8] Litvinov, Y. M.; Shestopalov, A. A.; Rodinovskaya, L. A.; Shestopalov, A. M. J. Comb. Chem. 2009, 11, 914.
[9] Vasuki, G.; Kumaravel, K. Tetrahedron Lett. 2008, 49, 5636.
[10] Ilovaisky, A. I.; Medvedev, M. G.; Merkulova, V. M.; Elinson, M. N.; Nikishin, G. I. J. Heterocycl. Chem. 2014, 51, 523.
[11] Zheng, J.; Li, Y. Mendeleev Commun. 2011, 21, 280.
[12] Zhao, L. Q.; Li, Y. Q.; Chen, L.; Zhou, B. Chin. J. Org. Chem. 2010, 30, 124 (in Chinese). (赵丽琴, 李毅群, 陈路, 周波, 有机化学, 2010, 30, 124.)
[13] Guo, R. Y.; An, Z. M.; Mo, L. P.; Yang, S. T.; Liu, H. X.; Wang, S. X.; Zhang, Z. H. Tetrahedron 2013, 69, 9931.
[14] Elnagdi, N. M. H.; Al-Hokbany, N. S. Molecules 2012, 17, 4300.
[15] Bihani, M.; Bora, P. P.; Bez, G.; Askari, H. ACS Sustainable Chem. Eng. 2013, 1, 440.
[16] Mecadon, H.; Rohman, M. R.; Rajbangshi, M.; Myrboh, B. Tetrahedron Lett. 2011, 52, 2523.
[17] Wang, X. S.; Shi, D. Q.; Tu, S. J.; Yao, C. S. Synth. Commun. 2003, 33, 119.
[18] Tu, S. J.; Gao, Y.; Guo, C.; Shi, D. Q.; Lu, Z. S. Synth. Commun. 2002, 32, 2137.
[19] (a) Leadbeater, N. E.; Marco, M. Chem. Rev. 2002, 102, 3217.
(b) Buchmeiser, M. R. Chem. Rev. 2008, 109, 303.
[20] (a) De Vos, D. E.; Dams, M.; Sels, B. F.; Jacobs, P. A. Chem. Rev. 2002, 102, 3615.
(b) Thomas, J. M.; Raja, R. Acc. Chem. Res. 2008, 41, 708.
[21] Oded, K.; Musa, S.; Gelman, D.; Blum, J. Catal. Commun. 2012, 20, 68.
[22] Abu-Reziq, R.; Shenglof, M.; Penn, L.; Cohen, T.; Blum, J. J. Mol. Catal. A: Chem. 2008, 290, 30.
[23] Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L. M.; Pagliaro, M. ChemCatChem 2015, 7, 254.
[24] Diz, P.; Pernas, P.; El Maatougui, A.; Tubio, C. R.; Azuaje, J.; Sotelo, E.; Guitián, F.; Gil, A.; Coelho, A. Appl. Catal. A: Gen. 2015, 502, 86.
[25] Bandgar, B. P.; Uppalla, L. S. Synth. Commun. 2000, 30, 2071.
[26] Chaphekar, S. S.; Samant, S. D. Appl. Catal. A: Gen. 2003, 242, 11.
[27] Shinde, S.; Rashinkar, G.; Kumbhar, A.; Kamble, S.; Salunkhe, R. Helv. Chim. Acta 2011, 94, 1943.
[28] Shinde, S.; Rashinkar, G.; Salunkhe, R. J. Mol. Liq. 2013, 178, 122.
[29] Jadhav, S.; Kumbhar, A.; Kamble, S.; More, P.; Salunkhe, R. C. R. Chim. 2013, 16, 957.
[30] Natekar, R.; Samant, S. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1996, 35, 1347.
[31] (a) Dippy, J. F. J.; Evans, R. M. J. Org. Chem. 1950, 15, 451.
(b) Li, Y. L.; Zhou, J. F.; Gong, G. X.; Zhu, F. X. Chin. J. Org. Chem. 2009, 29, 441 (in Chinese). (李艳伦, 周建峰, 贡桂霞, 朱凤霞, 有机化学, 2009, 29, 441.)
(c) Bian, Q. L.; Xu, S.; Duan, W. L. Chin. J. Org. Chem. 2015, 35, 234 (in Chinese). (边庆龙, 许胜, 段伟良, 有机化学, 2015, 35, 234.)
[32] Nasseri, M. A.; Sadeghzadeh, S. M. Monatsh. Chem. 2013, 144, 1551.
[33] Elinson, M. N.; Dorofeev, A. S.; Feducovich, S. K.; Gorbunov, S. V.; Nasybullin, R. F.; Miloserdov, F. M.; Nikishin, G. I. Eur. J. Org. Chem. 2006, 4335.
[34] Peng, Y.; Song, G.; Dou, R. Green Chem. 2006, 8, 573.
[35] Sharanina, L. G.; Promonenkov, V. K.; Marshtupa, V. P.; Pashchenko, A. V.; Puzanova, V. V.; Sharanin, Y. A.; Klyuev, N. A.; Gusev, L. F.; Gnatusina, A. P. Chem. Heterocycl. Compd. 1982, 18, 607.
/
| 〈 |
|
〉 |