

1,3-二取代-1H-1,2,4-三唑-5-胺类化合物的合成、结构表征及抑菌活性
收稿日期: 2015-10-19
修回日期: 2015-11-19
网络出版日期: 2015-12-10
基金资助
国家自然科学基金(No.31272076)和农业部行业专项基金(No.2011BAE06B05-5)资助项目.
Synthesis, Structure Characterization and Fungicidal Activity of 1,3-Disubstituted-1H-1,2,4-triazole-5-amines
Received date: 2015-10-19
Revised date: 2015-11-19
Online published: 2015-12-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 31272076) and the National Scientific and Technology Supporting Program of China (No. 2011BAE06B05-5).
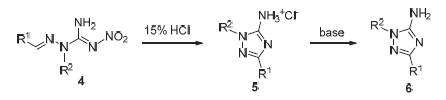
以硝基胍、醛和卤代烷烃为原料,通过肼解、缩合、烷基化和酸催化分子内环化等反应,合成了一系列结构新颖的1,3-二取代-1H-1,2,4-三唑-5-胺类化合物,其结构经1H NMR、13C NMR、IR以及HRMS等方法确证.初步离体抑菌活性测定表明,在50mg/L药剂浓度下,大部分化合物均具有一定的抑菌活性.相对于其他目标化合物,1-正丁基-3-(4-氯苯基)-1H-1,2,4-三唑-5-胺(6y)表现出较广谱的抑菌活性,对五种病原菌的抑菌活性均在53%以上.该类化合物合成简单,易于衍生化,可作为抑菌先导化合物进行优化.
关键词: 1H-1,2,4-三唑-5-胺; 合成; 分子内环化; 抑菌活性
贾长青 , 苏旺苍 , 徐彦军 , 刘吉平 , 覃兆海 . 1,3-二取代-1H-1,2,4-三唑-5-胺类化合物的合成、结构表征及抑菌活性[J]. 有机化学, 2016 , 36(4) : 830 -837 . DOI: 10.6023/cjoc201510021
A novel series of 1,3-disubstituted-1H-1,2,4-triazole-5-amines were synthesized via hydrazinolysis, condensation, alkylation and acid-catalyzed intramolecular cyclization starting from nitroguanidine 1. Their structures were characterized by IR, 1H NMR, 13C NMR and HRMS analysis. The preliminary bioassay indicated that most of them showed certain extent of growth inhibition in vitro against six phytopathogen fungi. Compared with other target compounds, 1-(n-butyl)-3- (4-chlorophenyl)-1H-1,2,4-triazole-5-amine (6y) was found to have high activity and the widest fungicidal spectrum. Its inhibition rates to five pathogens were all above 53%. This kind of compounds could be synthesized and derived easily, and were valuable as lead structure for future developing novel fungicides.

[1] Barbuceanu, S. F.; saramet, G.; Almajan, G. L.; Draghici, C.; Barbuceanu. F.; Bancescu, G. Eur. J. Med. Chem. 2012, 49, 417.
[2] Sato, T.; Ashizawa, N.; Iwanaga, T.; nakamura, H.; Matsumoto, K.; Inoue, T.; Nagata, O. Bioorg. Med.Chem. Lett. 2009, 19, 184.
[3] Papakonstantinou-Garoufalias, S.; Pouli, N.; marakos, P.; Chytyroglou-Ladas, A. Farmaco 2002, 57, 973.
[4] Abdel-Megeed, A. M.; Abdel-Rahman, H. M.; Alkaramany, G. S.; EI-Gendy, M. A. Eur. J. Med. Chem. 2009, 44, 117.
[5] Mallikarjuna, B. P.; Sastry, B. S.; Suresh Kumar, G. V.; Rajendraprasad, Y.; Chandrashekar, S. M.; Sathisha, K. Eur. J. Med. Chem. 2009, 44, 4739.
[6] Mange, Y. J.; Isloor, A. M.; Malladi, S.; Isloor, S. Arabian J. Chem. 2011, 6, 177.
[7] Zhang, R. B.; Lu, J. R.; Xin, C. W.; Liu, J. B.; Mu, J. B.; Yang, X. Y.; Wang, H. Y.; Wang, M. J.; Zhang, H. Chin. J. Org. Chem. 2015, 35, 858 (in Chinese). (张瑞波, 卢俊瑞, 辛春伟, 刘金彪, 穆江蓓, 杨旭云, 王宏韫, 王美君, 张贺, 有机化学, 2015, 35, 858.)
[8] Yu, G. P.; Xu, L. Z.; Yi, X.; Bi, W. Z.; Zhu, Q.; Zhai, Z. Z. J. Agric. Food Chem. 2009, 57, 4854.
[9] Arnoldi, A.; Dallavalle, S.; Merlini, L.; Musso, L.; Farina, G.; Moretti, M.; Jayasinghe, L. J. Agric. Food Chem. 2007, 55,8187.
[10] Ma, Y. M.; Liu, R. H.; Gong, X. Y.; Li, Z.; Huang, Q. C.; Wang, H. S.; Song, G. H. J. Agric. Food Chem. 2006, 54, 7724.
[11] Fan, Z. J.; Yang, Z. K.; Zhang, H. K.; Mi, N.; Wang, H.; Cai, F.; Zuo, X.; Zheng, Q. X.; Song, H. B. J. Agric. Food Chem. 2010, 58, 2630.
[12] Wang, H. Q.; Zhou, W. P.; Wang, Y. Y.; Lin, C. R. J. Agric. Food Chem. 2008, 56, 7321.
[13] Huang, W.; Zhao, P. L.; Liu, C. L.; Chen, Q.; Liu, Z. M.; Yang, G. F. J. Agric. Food Chem. 2007, 55, 3004.
[14] Cao, X.; Li, F.; Hu, M.; Lu, W.; Yu, G. A.; Liu, S. H. J. Agric. Food Chem. 2008, 56, 11367.
[15] Chen, X.; Yang, C. L. J. Agric. Food Chem. 2009, 57, 2441.
[16] Wang, B. L.; Shi, Y. X.; Ma, Y.; Liu, X. H.; Li, Y. H.; Song, H. B.; Li, B. J.; Li, Z. M. J. Agric. Food Chem. 2010, 58, 5515.
[17] Zhang, C. L.; Wang, X.; Guo, Y.; Wu, Y. F.; Gao, L. N.; Sun, L. J.; Chai, J. H.; Zhu, C. A. Chin. J. Org. Chem. 2014, 34, 2331 (in Chinese). (张成路, 王雪, 国阳, 吴一非, 高丽娜, 孙丽杰, 柴金华, 朱长安, 有机化学, 2014, 34, 2331.)
[18] Wang, B. L.; Shi, Y. X.; Zhan, Y. Z.; Zhang, L. Y.; Zhang, Y.; Wang, L. Z.; Zhang, X.; Li, Y. H.; Li, Z. M.; Li, B. J. Chin. J. Chem. 2015, 33, 1124.
[19] Su, W. C.; Zhou, Y. H.; Ma, Y. Q.; Wang, L.; Zhang, Z.; Rui, C. H.; Duan, H. X.; Qin, Z. H. J. Agric. Food Chem. 2012, 60, 5028.
[20] Yan, D. Y.; Wang, L.; Jia, C. Q.; Li, C. S.; Ma, Y. Q.; Rui, C. H.; Xu, Y. J.; Qin, Z. H. Chem. J. Chin. Univ. 2014, 35, 1703 (in Chinese).
/
| 〈 |
|
〉 |