

含1,2,4-三唑席夫碱的新型喹唑啉-2,4-二酮类衍生物的合成及其抗细菌活性研究
收稿日期: 2015-10-09
修回日期: 2015-11-05
网络出版日期: 2015-12-10
基金资助
国家自然科学基金(No.21362003)资助项目.
Synthesis and Antibacterial Activities of Novel Quinazoline-2,4-dione Derivatives Containing the 1,2,4-Triazole Schiff-Base Unit
Received date: 2015-10-09
Revised date: 2015-11-05
Online published: 2015-12-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21362003).
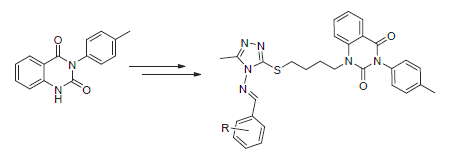
利用活性亚结构拼接原理,设计合成了18个含1,2,4-三唑席夫碱结构单元的喹唑啉-2,4-二酮类化合物7a~7r,通过1H NMR、13C NMR、MS、IR和元素分析对它们的结构进行了表征.初步抗菌测试结果表明,所有目标化合物在200μg/mL浓度下对水稻白叶枯病菌都表现出优良的抑制活性(≥93%),明显优于对照药剂噻菌铜和叶枯唑;其中化合物7a、7c~7g和7j~7l在100μg/mL浓度下对水稻白叶枯病菌的抑制率仍达100%.此外,所有目标化合物对柑橘溃疡病菌都表现出一定的抑制作用,但对烟草青枯病菌几乎无抑制活性.
关键词: 1,2,4-三唑席夫碱; 喹唑啉-2,4-二酮; 合成; 抗细菌活性
潘顶伍, 杜欢, 吕新阳, 鲍小平 . 含1,2,4-三唑席夫碱的新型喹唑啉-2,4-二酮类衍生物的合成及其抗细菌活性研究[J]. 有机化学, 2016 , 36(4) : 818 -825 . DOI: 10.6023/cjoc201510005
Eighteen novel quinazoline-2,4-dione derivatives containing the 1,2,4-triazole Schiff-base unit were designed and synthesized, based on the connection method of active fragments. Their structures were characterized by 1H NMR, 13C NMR, MS, IR and elemental analysis. The preliminary antibacterial test indicated that all the target compounds possessed an excellent inhibition activity (≥ 93%) against Xanthomonas oryzae pv. oryzae (Xoo) at the concentration of 200 μg/mL, which was obviously better than those of control agents thiodiazole-copper and bismerthiazol. Compounds 7a, 7c~7g and 7j~7l still displayed 100% inhibition rate against Xoo even at the concentration of 100 μg/mL. Moreover, all the target compounds exhibited a certain inhibition activity against Xanthomonas axonopodis pv. citri, but no inhibition activity against Ralstonia solanacearum.

[1] Dangi, R. R.; Chundawat, N. S.; Talesara, G. L. World J. Pharm. Res. 2015, 4, 1400.
[2] Wang, D.-W.; Lin, H.-Y.; Cao, R.-J.; Yang, S.-G.; Chen, Q.; Hao, G.-F.; Yang, W.-C.; Yang, G.-F. J. Agric. Food Chem. 2014, 62, 11786.
[3] Wang, D.; Lin H.; Cao, R.; Yang, S.; Chen, T.; He, B.; Chen, Q.; Yang, W.; Yang, G. Acta Chim. Sinica 2015, 73, 29 (in Chinese). (王大伟, 林红艳, 曹润洁, 杨盛刚, 陈涛, 何波, 陈琼, 杨文超, 杨光富, 化学学报, 2015, 73, 29.)
[4] Clark, R. L.; Clements, C. J.; Barrett, M. P.; Mackay, S. P.; Rathnam, R. P.; Owusu-Dapaah, G.; Spencer, J.; Huggan, J. K. Bioorg. Med. Chem. 2012, 20, 6019.
[5] El-Helby, A. G. A. Bull. Pharm. Sci., 2005, 28, 45.
[6] Colotta, V.; Lenzi, O.; Catarzi, D.; Varano, F.; Squarcialupi, L.; Costagli, C.; Galli, A.; Ghelardini, C.; Pugliese, A. M.; Maraula, G.; Coppi, E.; Pellegrini-Giampietro D. E.; Pedata, F.; Sabbadin, D.; Moro, S. Eur. J. Med. Chem. 2012, 54, 470.
[7] Yin, K.; Jiang, L.-H.; Zhou, H.-X.; Huang, Y.; Xiang, J.-N. Chin. J. Org. Chem. 2008, 28, 1016 (in Chinese). (尹凯, 蒋历辉, 周后相, 黄勇, 向建南, 有机化学, 2008, 28, 1016.)
[8] Xiong, Q.; Lin, X.; Liu, J.; Bi, L.; Bao, X. Chin. J. Org. Chem. 2012, 32, 1255 (in Chinese). (熊启中, 林选福, 刘军虎, 毕亮, 鲍小平, 有机化学, 2012, 32, 1255.)
[9] Krishna, K. M.; Inturi, B.; Pujar, G. V.; Purohit, M. N.; Vijaykumar, G. S. Eur. J. Med. Chem. 2014, 84, 516.
[10] Zhu, C.; Wu, F.; Wang, X.; Gao, L.; Weng, Q.; Shi, L.; Zhang, C. Chin. J. Appl. Chem. 2014, 31, 455 (in Chinese). (朱长安, 武飞宇, 王雪, 高丽娜, 翁前锋, 石丽, 张成路, 应用化学, 2014, 31, 455.)
[11] Liu, J.; Liu, Y.; Jian, J.; Bao, X. Chin. J. Org. Chem. 2013, 33, 370 (in Chinese). (刘军虎, 刘勇, 蹇军友, 鲍小平, 有机化学, 2013, 33, 370.)
[12] Li, C.; Liu, J.-C.; Li, Y.-R.; Gou, C.; Zhang, M.-L.; Liu, H.-Y.; Li, X.-Z.; Zheng, C.-J.; Piao, H.-R. Bioorg. Med. Chem. Lett. 2015, 25, 3052.
[13] He, C.; Chen, G. Plant Prot. 2010, 36, 6 (in Chinese). (何晨阳, 陈功友, 植物保护, 2010, 36, 6.)
[14] Taub, B.; Hino, J. B. J. Org. Chem. 1961, 26, 5238.
[15] Sun, X.-H.; Tao, Y.; Liu, Y.-F.; Jia, Y.-Q.; Chen, B. Acta Chim. Sinica 2008, 66, 234 (in Chinese). (孙晓红, 陶燕, 刘源发, 贾婴琦, 陈邦, 化学学报, 2008, 66, 234.)
[16] Wang, X.; Li, P.; Li, Z.; Yin, J.; He, M.; Xue, W.; Chen, Z.; Song, B. J. Agric. Food Chem. 2013, 61, 9575.
/
| 〈 |
|
〉 |