

新型萘酰亚胺-氟硼二吡咯荧光分子的合成、荧光共振能量转移及细胞成像
收稿日期: 2015-10-24
修回日期: 2015-12-01
网络出版日期: 2015-12-21
基金资助
国家自然科学基金(No.61178057)资助项目.
A Novel Fluorescent Dye Naphthalene Imide-Fluorine Boron Two Pyrrole: Synthesis, Fluorescence Resonance Energy Transfer and Cell Imaging
Received date: 2015-10-24
Revised date: 2015-12-01
Online published: 2015-12-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 61178057).
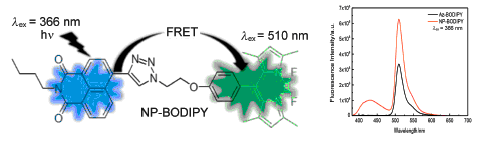
通过Click反应合成了萘酰亚胺-氟硼二吡咯复合结构荧光分子1-(2-(4-(1,3,5,7-四甲基氟硼二吡咯基)苯氧基)乙基)-4-(4-N-正丁基-1,8-萘酰亚胺)-1,2,3-三唑(NP-BODIPY),化合物结构经核磁共振氢谱、核磁共振碳谱以及高分辨质谱确征.NP-BODIPY存在从萘酰亚胺能量给体到氟硼二吡咯能量受体之间的分子内荧光共振能量转移.制备了负载萘酰亚胺-氟硼二吡咯荧光染料NP-BODIPY的二氧化硅荧光纳米粒子NP-BODIPY/SiO2,粒径为50nm,测定了NP-BODIPY的紫外可见吸收及荧光光谱.NP-BODIPY的固体在暗室中紫外灯下呈紫红色荧光;NP-BODIPY的THF溶液呈明亮的绿色荧光,荧光发射在430和510nm呈双峰结构,荧光量子产率为0.67,紫外吸收位于366和500nm;NP-BODIPY在含水量为80%H2O/THF混合溶液中的荧光较强,荧光量子产率为0.39,最大荧光峰位于510nm.将其与人乳腺癌细胞(MCF-7)共同孵化,荧光染料纳米粒子进入MCF-7细胞内并清晰成像.NP-BODIPY/SiO2荧光纳米粒子亲水性好,尺寸可控,细胞毒性低,生物相容性优,可广泛应用于生物标记及荧光成像.
沈宝星 , 钱鹰 . 新型萘酰亚胺-氟硼二吡咯荧光分子的合成、荧光共振能量转移及细胞成像[J]. 有机化学, 2016 , 36(4) : 774 -781 . DOI: 10.6023/cjoc201510028
A novel fluorescent dye naphthalene imide-fluorine boron two pyrrole 1-(2-(4-(1,3,5,7-tetramethyl fluorine boron pyrrole)phenoxy)ethyl)-4-(4-N-butyl-1,8-naphthalene imide)-1,2,3-triazole (NP-BODIPY) has been synthesized by Click reaction and characterized by 1H NMR, 13C NMR and HRMS techniques. NP-BODIPY has the property of intermolecule fluorescence resonance energy transfer from the naphthalene imide to BODIPY, the NP-BODIPY/SiO2nanoparticles was prepared by positive phase microemulsion. In the test of the UV-vis absorption and fluorescence spectrum of NP-BODIP, purple fluorescence could be observed under ultraviolet lamp when NP-BODIPY was in solid state, green fluorescence could be observed when NP-BODIPY was in THF solution, fluorescence spectrum exhibited bimodal structure in 430 and 510 nm, the fluorescence quantum efficiency of NP-BODIPY was 67% and the ultraviolet absorption peaks were at 366 and 500 nm. The fluorescence quantum efficiency of NP-BODIPY in H2O/THF mixture solution is 39%. It has strong fluorescence when water fraction is 80%, and the maximum fluorescence peaks are at 510 nm. When NP-BODIPY was co-incubated with michigan cancer foundation-7 cells (MCF-7) cells, the fluorescent dye penetrated into the MCF-7 cells and could be clearly observed. NP-BODIPY/SiO2 nanoparticles exhibit good water solubility, size controllable, low cytotoxicity and good biocompatibility. So NP-BODIPY can be widely used for biomarkers and fluorescence imaging.

[1] Wang, L.-Y.; Fang, G.; Cao, D. Sens. and Actuators B. 2015, 207, 849.
[2] He, X. G.; Zhang, J.; Liu, X. G.; Dong, L.; Li, D.; Qiu, H. Y.; Yin, S. C. Sens. Actuators B. 2014, 192, 29.
[3] Vedamalai, M.; Wu, S. P. Org. Biomol. Chem. 2012, 10, 5410.
[4] Zhang, X.-L. Ph. D. Dissertation, Dalian University of Technology, Dalian, 2009 (in Chinese). (张晓琳, 博士论文, 大连理工大学, 大连, 2009.)
[5] Zhu, L.; Younes, A.; Yuan, Z.; Clark, R. J. J. Photochem. Photobiol. A: Chem. 2015, 311, 1.
[6] Sreenath, K.; Yuan, Z.; Allen, J. R.; Davidson, M. W.; Zhu, L. Chem. Eur. J. 2015, 21, 867.
[7] Peng, X.; Du, J.; Fan, J. J. Am. Chem. Soc. 2007, 129, 500.
[8] Zhang, X.; Xiao, Y.; Qian, X. Org. Lett. 2008, 10, 29.
[9] Wang, J. M.; Yu, H.; Li, Q. Talanta 2015, 144, 763.
[10] Azael Gomez-Duran, C. F.; Hu, R. R.; Feng, G. X.; Li, T. Z.; Bu, F.; Arseneault, M.; Liu, Bin.; Peña-Cabrera, E.; Tang, B. Z. ACS Appl. Mater. Interfaces. 2015, 7, 15168.
[11] Zhao, C.; Jiang, H. Chin. J. Chem. 2015, 33, 7.
[12] Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057.
[13] Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem. 2002, 41, 2596.
[14] Hein, J. E.; Fokin, V. V. Chem. Soc. Rev. 2010, 39, 1302.
[15] Tron, G. C.; Pirali, T.; Billington, R. A.; Canonico, P. L.; Sorba, G.; Genazzani, A. A. Med. Res. Rev. 2008, 28, 278.
[16] Meldal, M.; Tornoe, C. W. Chem. Rev. 2008, 108, 2952.
[17] Bevilacqua, V.; King, M.; Chaumontet, M.; Nothisen, M.; Gabillet, S.; Buisson, D.; Puente, C.; Wagner, A.; Taran, F. Angew. Chem., Int. Ed. 2014, 53, 5872.
[18] Ingale, S. A.; Seela, F. J. Org. Chem. 2013, 78, 3394.
[19] Yuan, Z.; Kuang, G. C.; Clark, R. J.; Zhu, L. Org. Lett. 2012, 14, 2590.
[20] Cheng, H. R.; Qian, Y. RSC Adv. 2015, 5, 86371.
[21] Cheng, H. R.; Qian, Y. RSC Adv. 2015, 5, 82887.
[22] Mukherjee, S.; Thilagar, P. Chem. Eur. J. 2014, 20, 9052.
[23] Duke, R. M.; Gunnlaugsson, T. Tetrahedron Lett. 2007, 48, 8043.
[24] Zhu, L.; Yuan, Z.; J. Tyler, S.; Kesavapillai, S. RSC Adv. 2014, 4, 20398.
[25] Xu, Z.-C.; Juyoung, Y.; David, R. S. Chem. Soc. Rev. 2010, 39, 1996.
[26] Xuan-Anh, T.; Victor, A.; Paolo, B.; Bernadette, T. S. B.; Karsten, H. Biosens. Bioelectron. 2015, 64, 359.
[27] Gan, J.-A.; Song, Q.-L.; Hou, X.-Y.; Chen, K.-C.; Tian, H. J. Photochem. Photobiol. A 2004, 162, 399.
[28] Jin, P.-W.; Jiao, C.-H.; Guo, Z.-Q.; He, Y.; Zhu, S.-Q.; Tian, H.; Zhu, W.-H. Chem. Sci. 2014, 5, 4012.
[29] Liu, X.-L.; Du, X.-J.; Dai, C.-G.; Song, Q.-H. J. Org. Chem. 2014, 79, 9481.
[30] Liu, Y.-B.; Liu, Y.-W; Liu, W.; Liang, S.-C. Spectrochim. Acta A 2015, 137, 509.
[31] Sun, J.-F.; Qian, Y. Chin. J. Org. Chem. 2015, 35, 1104 (in Chinese). (孙京府, 钱鹰, 有机化学, 2015, 35, 1104.)
[32] Jin, Y.; Qian, Y. New J. Chem. 2015, 39, 2872.
[33] Huimin, G.; Muro-Small, M. L.; Shaomin, J. Inorg. Chem. 2010, 49, 6802.
[34] Cheng, H. R.; Qian, Y. Sens. Actuators, B 2015, 219, 57.
/
| 〈 |
|
〉 |