

功能化多取代噁唑类化合物的合成研究新进展
收稿日期: 2015-10-27
修回日期: 2015-12-20
网络出版日期: 2016-01-04
基金资助
河北省自然基金(No.B2013408014)和河北省教育厅(No.ZD2016046)资助项目.
Recent Development of Synthesis of Functionalized Polysubstituted Oxazoles
Received date: 2015-10-27
Revised date: 2015-12-20
Online published: 2016-01-04
Supported by
Project supported by the Natural Science Foundation of Hebei Province (No. B2013408014) and the Educational Commission of Hebei Province (No. ZD2016046).
肖立伟 , 张光霞 , 景学敏 , 周秋香 , 冯茹 . 功能化多取代噁唑类化合物的合成研究新进展[J]. 有机化学, 2016 , 36(5) : 1000 -1013 . DOI: 10.6023/cjoc201510032
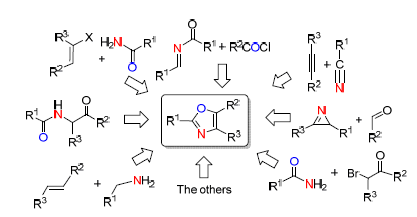
Functionalized multi-substituted oxazoles including di-and tri-substituted oxazoles, play important roles in the field of pharmaceutical, catalysis and supramolecular chemistry, etc. Based on different starting materials and different methods, the recent advances in synthesis of functionalized multi-substituted oxazoles are reviewed.
[1] Palmer, D. C.; Taylor, E. C. The Chemistry of Heterocyclic Compounds. Oxazoles: Synthesis, Reactions and Spectroscopy, Parts Wiley, Hoboken, NJ, 2004, Vol. 60.
[2] Yeh, V. S. C. Tetrahedron 2004, 60, 11995.
[3] Jin, Z. Nat. Prod. Rep. 2009, 26, 382.
[4] Hartmann-Thompson, C.; Keeley, D. L.; Pollock, K. M.; Dvornic, P. R.; Keinath, S. E.; Dantus, M.; Gunaratne, T.C.; LeCaptain, D. J. Chem. Mater. 2008, 20, 2829.
[5] Fuwa, H.; Naito, S.; Goto, T.; Sasaki, M. Angew. Chem. 2008, 120, 4815.
[6] Zhang, H. Z.; Zhou, C. H.; Geng, R. X.; Ji, Q. G. Chin. J. Org. Chem. 2011, 31, 1963 (in Chinese).
(张慧珍, 周成合, 耿蓉霞, 吉庆刚, 有机化学, 2011, 31, 1963.)
[7] Xiao, L.; Gao, H.; Kong, J.; Liu, G.; Peng, X.; Wang, S. Chin. J. Org. Chem.2014, 34, 1048 (in Chinese).
(肖立伟, 高红杰, 孔洁, 刘光仙, 彭晓霞, 王树军, 有机化学, 2014, 34, 1048.)
[8] Xiao, L.; Peng, X.; Zhou, Q.; Kou, W.; Shi, Y. Chin. J. Org. Chem.2015, 35, 1204 (in Chinese).
(肖立伟, 彭晓霞, 周秋香, 寇伟, 时亚茹, 有机化学, 2015, 35, 1204.)
[9] Xiao, L. Chin. J. Org. Chem. 2011, 31, 878 (in Chinese).
(肖立伟, 有机化学, 2011, 31, 878.)
[10] Zheng, M.; Huang, L.; Huang, H.; Li, X.; Wu, W.; Jiang, H. Org.Lett. 2014, 16, 5906.
[11] Shaw, A. Y.; Xu , Z.; Hulme, C. Tetrahedron Lett.2012, 53, 1998.
[12] Kumar, M. P.; Liu, R.-S. J. Org. Chem.2006, 71, 4951.
[13] Milton, M. D.; Inada, Y.; Nishibayashi, Y.; Uemura, S. Chem. Commun. 2004, 2712.
[14] Pan, Y.; Zheng, F.; Lin, H.; Zhan, Z. J. Org. Chem.2009, 74, 3148.
[15] Wipf, P.; Aoyama, Y.; Benedum, T. E.; Org. Lett. 2004, 6, 3593.
[16] Ritson, D. J.; Spiteri, C.; Moses, J. E. J. Org. Chem. 2011, 76, 3519.
[17] Gao, W.-C.; Wang, R.-L.; Zhang, C. Org. Biomol. Chem. 2013, 11, 7123.
[18] Zhao, Q.; Liu, S.; Li, Y.; Wang,Q. J. Agric. Food Chem.2009, 57,2849.
[19] Dinda, M.; Samanta, S.; Eringathodiab, S.; Ghosh, P. K. RSC Adv. 2014, 4, 12252.
[20] Thalhammer, A.; Mecinovic, J.; Schofield, C. J. Tetrahedron Lett.2009, 50, 1045.
[21] Giddens, A. C.; Boshoff, H. I. M.; Franzblau, S. G.; Barry III, C. E.; Coppa, B. R. Tetrahedron Lett. 2005, 46, 7355.
[22] Phillips, A. J.; Uto, Y.; Wipf, P.; Reno, M. J.; Williams, D. R. Org. Lett. 2000, 2, 1165.
[23] Chudasama, V.; Wilden, J. D.Chem. Commun. 2008, 3768.
[24] Tsai,Y.-L.; Fan,Y.-S.; Lee, C.-J.; Huang, C.-H.; Das, U.; Lin, W. Chem. Commun.2013, 49, 10266.
[25] Panda, N.; Mothkuri, R.New J. Chem.2014, 38, 5727.
[26] Zheng, Y.; Li, X.; Ren, C.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. J. Org. Chem.2012, 77, 10353.
[27] Fan, Y.-S.; Das, U.; Hsiao, M.-Y.; Liu, M.-H.; Lin, W. J. Org. Chem. 2014, 79, 11567.
[28] Martín, R.; Cuenca, A.; Buchwald, S. L. Org. Lett. 2007, 9, 5521.
[29] Xu, W.; Kloeckner, U.; Nachtsheim, B. J. J. Org. Chem.2013, 78, 6065.
[30] Morwick, T.; Hrapchak, M.; DeTuri, M.; Campbell, S. Org. Lett. 2002, 4, 2665.
[31] Zhang, J.; Coqueron, P.-Y.; Vors, J.-P.; Ciufolini, M. A. Org. Lett.2010, 12, 3942
[32] Spencer, J.; Patel, H.; Amin, J.; Callear, S. K.; Coles, S. J.; Deadman, J. J.; Furman, C.; Mansouri, R.; Chavatte, P.; Millet,R. Tetrahedron Lett. 2012, 53, 165.
[33] Huang, W.; Dong, G.; Mijiti,Z.Tetrahedron2012, 68, 977.
[34] Pulici, M.; Quartieri, F.; Felder, E. R. J. Comb. Chem.2005, 7,463.
[35] Graham, T. H. Org. Lett.2010, 12, 3614.
[36] Glöckner, S.; Tran, D. N.; Ingham, R. J.; Fenner, S.; Wilson, Z. E.; Battilocchio, C.; Ley, S. V. Org. Biomol. Chem. 2015, 13, 207.
[37] Wan, C.; Zhang, J.; Wang, S.; Fan, J.; Wang, Z.Org. Lett.2010, 12,2338.
[38] Xie, J.; Jiang, H.; Cheng, Y.; Zhu, C. Chem. Commun.2012, 48, 979.
[39] Naresh, G.; Narende, T. RSC Adv. 2014, 4, 11862.
[40] Yuan, G.; Zhu, Z.; Gao, X.; Jiang, H. RSC Adv. 2014, 4, 24300.
[41] Hu, P.; Wang, Q.; Yan, Y.; Zhang, S.; Zhang, B.; Wang, Z. Org. Biomol. Chem. 2013, 11, 4304.
[42] Patil, P. C.; Luzzio, F. A.; Demuth, D. R.Tetrahedron Lett. 2015, 56, 3039.
[43] Sun, J.; Zhang, F.;Li, Y.;Gao, D.;Zhang, Y.;Zhao, T.; Chen, X.Chin. J. Org. Chem.2008, 28, 1611 (in Chinese).
(孙继奎,张峰,李亚卓,高大维, 张玉敏,赵天琦,陈晓东, 有机化学, 2008, 28, 1611.)
[44] Liu, X.; Cheng, R.; Zhao, F.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett.2012, 14, 5480.
[45] Kawano, Y.; Togo, H. Tetrahedron 2009, 65, 6251.
[46] Ishiwata, Y.; Togo, H. Tetrahedron 2009, 65, 10720.
[47] Saito, A.; Taniguchi, A.; Kambara, Y.; Hanzawa, Y. Org. Lett. 2013, 15, 2672.
[48] Herrera, A.; Martínez-Alvarez, R.; Ramiro, P.; Molero, D.; Almy, J. J. Org. Chem.2006, 71, 3026.
[49] Baumann, M.; Baxendale, I. R.; Ley, S. V.; Smith, C. D.; Tranmer, G. K. Org. Lett. 2006, 8, 5231.
[50] Wang, Q.; Ganem, B. Tetrahedron Lett. 2003, 44, 6829.
[51] Senadi, G. C.; Hu, W.-P.; Hsiao, J.-S.; Vandavasi, J. K.; Chen, C.-Y.; Wang, J.-J. Org. Lett. 2012, 14, 4478.
[52] Chatzopoulou, E.; Davies, P. W. Chem. Commun.2013, 49, 8617.
[53] Hashmi, A. S K.; Weyrauch, J. P.; Frey, W.; Bats, J. W. Org. Lett. 2004, 6, 4391.
[54] Wachenfeldt, H.; Rǒse, P.; Paulsen, F.; Loganathan, N.; Strand, D. Chem. Eur. J. 2013, 19, 7982.
[55] Wachenfeldt, H.; Polukeev, A. V.; Loganathan, N.; Paulsen, F.; Röse, P.; Garreau, M.; Wendt, O. F.; Strand, D. Dalton Trans.2015, 44, 5347.
[56] He, W.; Li, C.; Zhang, L. J. Am. Chem. Soc.2011, 133, 8482.
[57] Hu, Y.; Yi, R.; Wu, F.; Wan, B. J. Org. Chem.2013, 78, 7714.
[58] Zhang, L.; Zhao, X. Org. Lett. 2015, 17, 184.
[59] Zheng, J.; Zhang, M.; Huang, L.; Hu, X.; Wu, W.; Huang, H.; Jiang, H. Chem. Commun.,2014, 50, 3609.
[60] Zhao, F.; Liu, X.; Qi, R.; Zhang-Negrerie, D.; Huang, J.; Du, Y.; Zhao, K.J. Org. Chem. 2011, 76, 10338.
[61] Xie, H.; Yuan, D.; Ding, M.-W. J. Org. Chem. 2012, 77, 2954.
[62] Jiang, H.; Huang, H.; Cao, H.; Qi, C. Org. Lett. 2010, 12,5561.
[63] Shi, B.; Blake, A. J.; Lewis, W.; Campbell, I. B.; Judkins, B. D.; Moody, C. J.J. Org. Chem. 2010, 75, 152.
[64] Xu, X.; Zavalij, P. Y.; Hu, W.; Doyle, M. P. Chem. Commun.2012, 48, 11522.
[65] Murali Dhar, T. G.; Guo, J.; Shen, Z.; Pitts, W. J.; Gu, H. H.; Chen, B.-C.; Zhao, R.; Bednarz, M. S.; Iwanowicz, E. J. Org. Lett. 2002, 4, 2091.
[66] Keni, M.; Tepe, J. J. J. Org. Chem.2005, 70, 4211.
[67] Zhong, C. L.; Tang, B. Y.; Yin, P.; Chen, Y.; He, L. J. Org. Chem.2012, 77, 4271.
[68] Misra, N. C.; Ila, H. J. Org. Chem. 2010, 75, 5195.
[69] Kumar, S. V.; Saraiah, B.; Misra, N. C.; Ila, H. J. Org. Chem. 2012, 77, 10752.
[70] Hodgetts, K. J.; Kershaw,M. T.Org. Lett. 2002, 4, 2905.
[71] Miller, R. A.; Smith, R. M.; Marcune, B. J. Org. Chem.2005, 70, 9074.
[72] Tabuchi, S.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2014, 79, 5401.
[73] Ohnmacht, S. A.; Mamone, P.; Culshawb, A. J.; Greaney, M. F. Chem. Commun. 2008, 1241.
[74] Lee, K.; Counceller, C. M.; Stambuli, J. P. Org. Lett.2009, 11, 1457.
[75] Langille, N. F.; Dakin, L. A.; Panek, J. S. Org. Lett.2002, 4, 2485.
[76] Hoffman, T. J.; Rigby, J. H.; Arseniyadis, S.; Cossy, J. J. Org. Chem.2008, 73, 2400.
[77] Selvi, T.; Srinivasan, K. Chem. Commun.2014, 50, 10845.
[78] Zeng, T.-T.; Xuan,J.; Ding, W.; Wang, K.; Lu, L.-Q.; Xiao, W.-J. Org. Lett. 2015, 17, 4070.
/
| 〈 |
|
〉 |