

新型含喹啉酮结构的三唑磺酰胺衍生物的合成及其对线粒体复合物III的抑制活性
收稿日期: 2015-10-12
修回日期: 2015-11-30
网络出版日期: 2016-01-07
基金资助
国家自然科学基金(No.21272091)、湖北省教育厅项目(No.Q20102606)、中央高校基本科研业务费(No.CCNU15A02014)、襄阳市科技局项目(No.2010GG1B33)、湖北省自然科学基金(No.2014CFC1153)资助项目.
Synthesis and Biological Activities of Novel Triazolesulfonamide Derivatives Containing Quinolin-2-one Moiety
Received date: 2015-10-12
Revised date: 2015-11-30
Online published: 2016-01-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272091), the Hubei Provincial Department of Education (No. Q20102606), the Fundamental Research Funds for the Central Universities (No. CCNU15A02014), the Xiangyang Science and Technology Bureau (No. 2010GG1B33), and the Natural Science Foundation of Hubei Province (No. 2014CFC1153).
线粒体复合物III是细胞呼吸链的重要组成部分,也是杀菌剂的重要作用靶标之一.根据生物电子等排、活性亚结构拼接原理,设计并合成了一系列含喹啉酮结构三唑磺酰胺衍生物,经质谱、氢谱、碳谱及元素分析对其结构进行了确认,测试了该类化合物对猪心线粒体复合物III的抑制活性,结果表明目标化合物6f[IC50=(45.75±1.02)μmol/L]对复合物III的抑制活性较对照药剂吲唑磺菌胺[IC50=(92.96±1.30)μmol/L]提高了一倍多,可作为先导结构进一步优化.
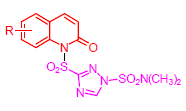
关键词: 2-喹啉酮; 1,2,4-三唑磺酰胺; 琥珀酸-细胞色素c氧化还原酶; 抑制活性; 合成
程华 , 汪万强 , 黄琳 , 崔萍 , 吴琼友 . 新型含喹啉酮结构的三唑磺酰胺衍生物的合成及其对线粒体复合物III的抑制活性[J]. 有机化学, 2016 , 36(5) : 1065 -1072 . DOI: 10.6023/cjoc201510013
Complex III is an essential component for cellular respiration chain and one of the most important targets of fungicides. According to bioisosterism and combination of bioactive sub-structive, a series of novel triazolesulfonamide derivatives containing quinolin-2-one moiety were designed and synthesized. The structures of the synthesized compounds were confirmed by MS, 1H NMR, 13C NMR and elemental analysis. The antibacterial activities of these compounds against porcine complex III were evaluated, and the bioassay results indicated that the target compound 6f [IC50=(45.75±1.02) μmol/L] showed much higher inhibitory potency (more than one-fold higher) against complex III than that of the control amisulbrom [IC50=(92.96±1.30) μmol/L], thus compound 6f may recognize as a lead for further structure modification.

[1] (1) Sun, F.; Zhou, Q. J.; Sun, J.; Zhai, Y. J.; Rao, Z. H. Bull. Life Sci.2008, 20(4), 566 (in Chinese).
(孙飞, 周强军, 孙吉, 翟宇佳, 饶子和, 生命科学, 2008, 20(4), 566.)
[2] Zhang, Z. L.; Lishar, H.; Shulmeister, V. M.; Chi, Y. I.; Kyeong, K. K.; Hung, L. W.; Crofts, A. R.; Berry, E. A.; Kim, S. H. Nature 1998, 392, 677.
[3] Iwata, S.; Lee, J. W.; Okada, K.; Lee, J. K.; Iwata, M.; Rasmussen, B.; Link, T. A.; Ramaswamy, S. Science 1998, 281, 64.
[4] Hunte, C.; Koepke, J.; Lange, C.; Romanith, T.; Michel, H. Structure 2000, 8(6), 669.
[5] Gao, X. G; Wen, X. L.; Esser, L.; Quinn, B.; Yu, L. D.; Yu, C. A.; Xia, D. Biochemistry 2003, 42(30), 9067.
[6] Esser, L.; Quinn, B.; Li, Y. F.; Zhang, M. M.; Elberry, L.; Yu, C. A.; Xia, D. J. Mol. Biol. 2004, 341(1), 281.
[7] Huang, L.; Cobessi, D.; Tung, E. Y.; Berry, E. A. J. Mol. Biol. 2005, 351(3), 573.
[8] Yang, W. C.; Li, H.; Wang, F.; Zhu, X. L.; Yang, G. F. ChemBioChem 2012, 13(11), 1542.
[9] Maklashina, E.; Cecchini, G. Biochim. Biophys. Acta 2010, 1797(12), 1877.
[10] Li, H.; Zhu, X. L.; Yang, W. C.; Yang, G. F. Chem. Biol. Drug. Des. 2014, 83(1), 71.
[11] Cheng, H.; Shen, Y. Q.; Pan, X. Y.; Hou, Y. P.; Wu, Q. Y.; Yang, G. F. New J. Chem. 2015, 39(9), 7281.
[12] Tang, J. L.; Xu, J. G.; Song, J.; Guang, L.; Chen, L. J. Guangdong Pharm. Univ. 2013, 29(6), 596 (in Chinese).
(汤洁丽, 许俊耿, 宋健, 关丽, 陈琳, 广东药学院学报, 2013, 29(6), 596.)
[13] Harper, S.; Avolio, S. J. Med. Chem. 2005, 48(2), 547.
[14] Oshiro, Y.; Sam, S. J. Med. Chem. 1998, 41(5), 658.
[15] Salmon, R.; Mitchell, G.; Morris, J. A. WO 2010116122, 2010[Chem. Abstr. 2010, 153, 470191].
[16] Yang, J. Q.; Hu, Y. W.; Gu, Q.; Li, M. G.; Li, M. Q.; Song, B. A. Chin. J. Org. Chem. 2014, 34, 829.
(in Chinese) (杨家强, 胡月维, 谷晴, 李明刚, 李明强, 宋宝安, 有机化学, 2014, 34, 829.)
[17] Mahieu, J. P.; Gosselet, M.; Sebille, B.; Beuzard, Y. Synth. Commun. 1986, 16(13), 1709.
[18] Manimaran, T.; Thiruvengadam, T. K.; Ramakrishnan, V. T. Synth. Commun. 1975, 11, 739.
[19] Hegde, S. G.; Mahoney, M. D. WO 2000042028, 2000[Chem. Abstr.2000, 133, 105038].
[20] Fukuda, K.; Kondo, Y.; Makabe, T.; Tanaka, N.; Utsunomiya, T.; Sakurai, Y. WO 2006057354, 2006[Chem. Abstr.2006, 145, 27993].
/
| 〈 |
|
〉 |