

α-重氮膦酸酯研究进展
收稿日期: 2015-11-12
修回日期: 2015-12-16
网络出版日期: 2016-01-07
基金资助
天津市自然科学基金(No.15JCYBJC20700)和新疆特色药食用植物资源化学实验室开放课题(No.2015KL030)资助项目.
Research Progress of α-Diazophosphonates
Received date: 2015-11-12
Revised date: 2015-12-16
Online published: 2016-01-07
Supported by
Project supported by the Committee of Science and Technology of Tianjin City (No. 15JCYBJC20700) and the Xinjiang Laboratory of Native Medicinal and Edible Plant Resources Chemistry Open Subject (No. 2015KL030).
苗志伟, 蔡岩, 葛海红, 付嘉欣, 木尼热·阿布都克力木 . α-重氮膦酸酯研究进展[J]. 有机化学, 2016 , 36(5) : 976 -986 . DOI: 10.6023/cjoc201511021
Diazo compounds are the most commonly used carbene precursors. They can be dediazonized to obtain highly reactive free carbene intermediates or metal cabenoid under transition metal catalysts. Then varieties of chemical transformations can be proceeded, such as X—H (X=C, N, O, S, Si, etc.) insertions, 1,2-hydrogen migration reactions and cyclopropanations. Varieties of pharmaceuticals, natural products and other bioactive moleculars could be synthesized through these methods. As one of the most important diazo compounds α-diazophosphonates could also proceed various chemical transformations and be used to synthesize varieties of organic functional phosphorous compounds. Because organic phosphorous compounds exhibit extensive bioactivities and pharmaceutic activities, the research of α-diazophosphonates has attracted lots of attentions of scientists. The recent development of the reactions of α-diazophosphonates catalyzed by various kinds of catalysts is summarized.
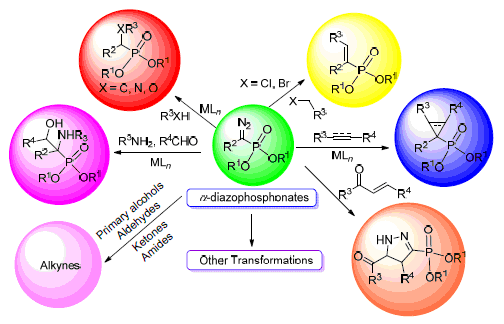
Key words: diazo compound; α-diazophosphonate; reaction property
[1] (a) Palacios, F.; Alonso, C.; de los Santos, J. M. Chem. Rev. 2005, 105, 899.
(b) Palacios, F.; Alonso, C.; de los Santos, J. M. In Enantioselective Synthesis of β-Amino Acids, 2nd ed., Eds.: Juaristi, E.; Soloshonok, V. A., Wiley, New York, 2005, pp. 277~317.
[2] (a) Zollinger, H. Diazo Chemistry I and II, VCH, Weinheim, 1994.
(b) Kurti, L.; Czako, B. Strategic Applications of Named Reactions in Organic Synthesis, Elsevier, Amsterdam, 2005, pp. 376~377.
(c) Regitz, M.; Maas, G. Diazo Compounds-Properties and Synthesis, Academic Press, Orlando, 1986.
[3] (a) Wang, J.; Boyarskikh, V.; Rainier, J. D. Org. Lett. 2011, 13, 700.
(b) Doyle, M. P.; Yan, M.; Hu, W.; Gronenberg, L. S. J. Am. Chem. Soc. 2003, 125, 4692.
(c) Lian, Y. J.; Davies, H. M. L. J. Am. Chem. Soc. 2011, 133, 11940.
(d) Wang, X. C.; Xu, X. F.; Zavalij, P. Y.; Doyle, M. P. J. Am. Chem. Soc. 2011, 133, 16402.
(e) Briones, J. F.; Davies, H. M. L. J. Am. Chem. Soc. 2013, 133, 13314.
(f) Smith, A. G.; Davies, H. M. L. J. Am. Chem. Soc. 2012, 134, 18241.
[4] Cox, G. G.; Miller, D. J.; Moody, C. J.; Robert, E.; Sie, H. B. Tetrahedron 1994, 50, 3195.
[5] Gois, P. M. P.; Afonso, C. A. M. Eur. J. Org. Chem. 2003, 3798.
[6] Candeias, N. R.; Gois, P. M. P.; Afonso, C. A. M. J. Org. Chem. 2006, 71, 5489.
[7] Candeias, N. R.; Gois, P. M. P.; Veiros, L. F.; Afonso, C. A. M. J. Org. Chem. 2008, 73, 5926.
[8] Zhu, S. F.; Chen, W. Q.; Zhang, Q. Q.; Mao, H. X.; Zhou, Q. L. Synlett2011, 919.
[9] Hladeuk, I.; Chastagner, V.; Collins, S. G.; Plunkett, S. J.; Ford, A.; Debarge, S.; Maguire, A. R. Tetrahedron 2012, 68, 1894.
[10] (a) Davis, F. A.; Wu, Y. Z.; Xu, H.; Zhang, J. Y. Org. Lett. 2004, 6, 4523.
(b) Titanyuk, I. D.; Vorob'eva, D. V.; Osipov, S. N.; Beletskaya, I. P. Synlett2006, 1355.
[11] (a) Ukita, T.; Nakamura, Y. Org. Lett. 2002, 4, 2317.
(b) Haigh, D. Tetrahedron 1994, 50, 3177.
[12] (a) Xue, J. D.; Luk, H. L.; Platz, M. S. J. Am. Chem. Soc. 2011, 133, 1763.
(b) Zhu, S. F.; Xu, B.; Wang, G. P.; Zhou, Q. L. J. Am. Chem. Soc. 2012, 134, 436.
[13] Nakamura, E.; Yoshikai, N.; Yamanaka, M. J. Am. Chem. Soc. 2002, 124, 7181.
[14] (a) Salaun, J. Chem. Rev. 1989, 89, 1247.
(b) Donaldson, W. A. Tetrahedron 2001, 57, 8589.
(c) Faust, R. Angew. Chem., Int. Ed. 2001, 40, 2251.
(d) Pietruszka, J. Chem. Rev. 2003, 103, 1051.
(e) Wessjohann, L. A.; Brandt, W.; Thiemann, T. Chem. Rev. 2003, 103, 1625.
(f) Brackmann, F.; de Meijere, A. Chem. Rev. 2007, 107, 4493.
(g) Marek, I.; Simaan, S.; Masarwa, A. Angew. Chem., Int. Ed. 2007, 46, 7364.
(h) Rubin, M.; Rubina, M.; Gevorgyan, V. Chem. Rev. 2007, 107, 3117.
[15] (a) Schnaars, C.; Hansen, T. Org. Lett. 2012, 14, 2794.
(b) Schnaars, C.; Hennum, M.; Hansen, T. J. Org. Chem. 2013, 78, 7488.
[16] (a) Lindsay, V. N. G.; Fiset, D.; Gritsch, P. J.; Azzi, S.; Charette, A. B. J. Am. Chem. Soc. 2013, 135, 1463.
(b) Marcoux, D.; Goudreau, S. R.; Charette, A. B. J. Org. Chem. 2009, 74, 8939.
(c) Lifchits, O.; Charette, A. B. Org. Lett. 2008, 10, 2809.
(d) Pohlhaus, P. D.; Johnson, J. S. J. Am. Chem. Soc. 2005, 127, 16014.
(e) Campbell, M. J.; Johnson, J. S. J. Am. Chem. Soc. 2008, 131, 10370.
(f) Young, I. S.; Kerr, M. A. J. Am. Chem. Soc. 2007, 129, 1465.
[17] Briones, J. F.; Davies, H. M. L. Org. Lett. 2011, 13, 3984.
[18] (a) Jiang, J.; Xu, H. D.; Xi, J. B.; Ren, B. Y.; Lv, F. P.; Guo, X.; Jiang, L. Q.; Zhang, Z. Y.; Hu, W. H. J. Am. Chem. Soc. 2011, 133, 10370.
(b) Xing, D.; Hu, W. H. Tetrahedron 2014, 55, 777.
(c) Zhu, Y. G.; Zhai C. W.; Yang, L. P.; Hu, W. H. Eur. J. Org. Chem. 2011, 1113.
(d) Huang, H. X.; Guo, X.; Hu, W. H. Angew. Chem., Int. Ed. 2007, 46, 1337.
(e) Jing, C. C.; Xing, D.; Qian, Y.; Shi, T. D.; Zhao, Y.; Hu, W. H. Angew. Chem., Int. Ed. 2013, 52, 9289.
(f) Zhang, D.; Qiu, H.; Jiang, L. Q.; Lv, F. P.; Ma, C. Q.; Hu, W. H. Angew. Chem., Int. Ed. 2013, 52, 13356.
(g) Zhou, C. Y.; Wang, J. C.; Wei, J. H.; Xu, Z. J.; Guo, Z.; Low, K. H.; Che, C. M. Angew. Chem., Int. Ed. 2012, 51, 11376.
[19] Zhou, Y. J.; Ye, F.; Wang, X.; Xu, S.; Zhang, Y.; Wang, J. B. J. Org. Chem. 2015, 80, 6109.
[20] (a) Taber, D. F.; Herr, R. J.; Pack, S. K. J. Org. Chem. 1996, 61, 2908.
(b) Zhang, Z. H.; Wang, J. B. Tetrahedron 2008, 64, 6577.
(c) Jiang, N.; Ma, Z. H.; Qu, Z. H.; Xing, X. Y.; Xie, L. F.; Wang. J. B. J. Org. Chem. 2003, 68, 893.
(d) Zhou, L.; Liu, Y. Z.; Zhang, Y.; Wang, J. B. Chem. Commun. 2011, 47, 3622.
(e) Xu, F.; Zhang, S. W.; Wu, X. N.; Liu, Y.; Shi, W. F.; Wang, J. B. Org. Lett. 2006, 8, 3207.
(f) Xiao, F. P.; Wang, J. B. J. Org. Chem. 2006, 71, 5789.
(g) Xu, F.; Shi, W. F.; Wang, J. B. J. Org. Chem. 2005, 70, 4191.
(h) Jiang, N; Qu, Z. H.; Wang, J. B. Org. Lett. 2001, 3, 2989.
[21] Cai, Y.; Ge, H. H.; Yu, C. B.; Sun, W. Z.; Zhan, J. C.; Miao, Z. W. RSC Adv. 2014, 4, 1492.
[22] Ge, H. H.; Liu, S.; Cai, Y.; Sun, Y. C.; Miao, Z. W. Synthesis 2016, 48, 448.
[23] Cai, Y.; Lyu, H. R.; Yu, C. B.; Miao, Z. W. Adv. Synth. Catal. 2014, 356, 596.
[24] (a) Patil, U. D. Synlett 2009, 17, 2880.
(b) Gong, D. H.; Zhang, L.; Yuan, C. Y. Synth. Commun. 2004, 34, 3259.
(c) Bartnik, R.; Lesniak, S.; Wasiak, P. Tetrahedron Lett. 2004, 45, 7301.
(d) Mukund, M. D.; Pramanik, A.; Chaturvediab, K.; Rastogi, N. Chem. Commun. 2014, 50, 12896.
(e) Muruganantham, R.; Namboothiri, I. J. Org. Chem. 2010, 75, 2197.
(f) Muruganantham, R.; Mobin, S. M.; Namboothiri, I. N. N. Org. Lett. 2007, 9, 1125.
[25] Verma, D.; Mobin, S.; Namboothiri, I. N. N. J. Org. Chem. 2011, 76, 4764.
[26] Mohanan, K.; Martin, A. R.; Toupet, L.; Smietana, M.; Vasseur, J. J. Angew. Chem., Int. Ed. 2010, 49, 3196.
[27] Cai, Y.; Lu, Y. C.; Yu, C. B.; Lyu H. R., Miao, Z. W. Org. Biomol. Chem. 2013, 11, 5491.
[28] Cai, Y.; Ge, H. H.; Sun, W. Z.; Miao, Z. W. Synthesis2015, 47, 1669.
[29] Hu, C. F.; Cai, Y.; Munira, A.; Miao, Z. W. Chin. J. Org. Chem. 2015, 35, 2135 (in Chinese).
(胡辰飞, 蔡岩, 木尼热·阿布都克力木, 苗志伟, 有机化学, 2015, 35, 2135.)
/
| 〈 |
|
〉 |