

离子液体/相转移催化剂促进的二茂铁基查尔酮的合成
收稿日期: 2015-11-25
修回日期: 2015-12-14
网络出版日期: 2016-01-15
基金资助
国家自然科学基金(No. 21562032)、内蒙古自治区高等学校科学研究(No. NJZZ001)和内蒙古自治区自然科学基金(Nos. 2013MS0207, 2014JQ02)资助项目.
Facile Synthesis of Ferrocenylchalcone Promoted by Ionic Liquid or Phase-Transfer Catalyst
Received date: 2015-11-25
Revised date: 2015-12-14
Online published: 2016-01-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21562032), the Research Program of Science and Technology at Universities of Inner Mongolia (No. NJZZ001) and the Natural Science Foundation of Inner Mongolia (Nos. 2013MS0207, 2014JQ02).
乙酰基二茂铁与芳香醛在离子液体或相转移催化剂作用下进行Claisen-Schmidt缩合,高产率地合成二茂铁基查尔酮.其中,相转移催化剂能更有效地催化该反应,优化后的反应条件为:无水乙醇溶液,35℃,n(乙酰基二茂铁):n(芳香醛):n(四丁基六氟磷酸铵):n(NaOH)=1:1:2.5:0.75.
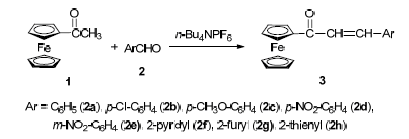
关键词: 二茂铁; 查尔酮; 离子液体; 相转移催化剂; Claisen-Schmidt缩合
赵海英 , 尹凤楠 , 于玲岩 , 李保国 , 边占喜 . 离子液体/相转移催化剂促进的二茂铁基查尔酮的合成[J]. 有机化学, 2016 , 36(5) : 1118 -1121 . DOI: 10.6023/cjoc201511045
The Claisen-Schmidt condensation of acetylferrocene with arylaldehydes in the presence of ionic liquids (ILs) or phase-transfer catalysts (PTCs) provided the corresponding ferrocenylchalcones in high yields, and PTCs can promote the condensation reaction better than those of ILs. Optimum conditions were as follows: molar ratio of acetylferrocene, arylaldehyde, n-Bu4NPF6 and NaOH is 1:1:2.5:0.75, and the appropriate reaction temperature is 35 ℃ in anhydrous EtOH.
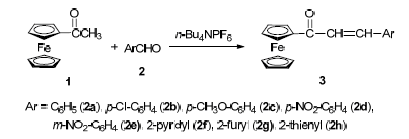
[1] Patil, P. S.; Dharmaprakash, S. M.; Ramakrishna, K.; Fun, H. K. J. Cryst. Growth 2007, 303, 520.
[2] Zhao, H.; Zhu, X.; Wang, D.; Chen, S.; Bian, Z. Aust. J. Chem. 2015, 68, 1035.
[3] Avila, H.; Smania, E.; Monache, F.; Junior, A. Bioorg. Med. Chem. 2008, 16, 9790.
[4] Niu, C.; Li, G.; Tuerxuntayi, A.; Aisa, H. A. Chin. J. Chem. 2015, 33, 486.
[5] Prasath, R.; Bhavana, P.; Ng, S. W.; Tiekink, E. R. T. J. Organomet. Chem. 2013, 726, 62.
[6] Attar, S.; O'Brien, Z.; Alhaddad, H.; Golden, M. L.; Calderón-Urrea, A. Bioorg. Med. Chem. 2011, 19, 2055.
[7] Wu, X.; Tiekink, E. R. T.; Kostetski, I.; Kocherginsky, N.; Tan, A. L. C.; Khoo, S. B.; Wilairat, P.; Go, M. L. Eur. J. Pharm. Sci. 2006, 27, 175.
[8] Wang, D.; Zhu, X. Y.; Zhao, H. Y.; Bian, Z. X. Chin. J. Org. Chem. 2015, 35, 1131 (in Chinese).
(王栋, 朱学友, 赵海英, 边占喜, 有机化学, 2015, 35, 1131.)
[9] Yang, J. M.; Ji, S. J.; Gu, D. G.; Shen, Z. L.; Wang, S. Y. J. Organomet. Chem. 2005, 690, 2989.
[10] Parveen, H.; Hayat, F.; Salahuddin, A.; Azam, A. Eur. J. Med. Chem. 2010, 45, 3497.
[11] Gong, Z. L.; Xie, Y. S; Zhao, B. X.; Lv, H. S.; Liu, W. Y.; Zheng, L. W.; Lian, S. J. Fluoresc. 2011, 21, 355.
[12] Bukhari, S. N. A.; Jasamai, M.; Jantan, I.; Ahmad, W. Mini-Rev. Org. Chem. 2013, 10, 73.
[13] Fang, D.; Cheng, J.; Fei, Z.; Gong, K.; Liu, Z. Catal. Commun. 2008, 9, 1924.
[14] Shibata, K.; Katsuyama, I.; Matsui, M.; Muramatsu, H. Bull. Chem. Soc. Jpn. 1990, 63, 3710.
[15] Erasmus, E. Inorg. Chim. Acta 2011, 378, 95.
[16] Abashev, G. G.; Antuf'eva, A. D.; Bushueva, A. Y.; Kudryavtsev, P. G.; Osorgina, I. V.; Syutkin, R. V.; Shklyaeva, E. V. Russ. J. Appl. Chem. 2010, 83, 1435.
[17] Muller, T. J.; Conradie, J.; Erasmus, E. Polyhedron 2012, 33, 257.
[18] Srivastava, Y. K. Rasayan J. Chem. 2008, 1, 884.
[19] Liu, Y. T.; Lian, G. D.; Yin, D. W.; Su, B. J. Res. Chem. Intermed. 2012, 38, 1043.
[20] Liu, Y. T.; Feng, L.; Yin, D. W. Res. Chem. Intermed. 2013, 39, 2971.
[21] Ji, S. J.; Wang, S. Y.; Shen, Z. L.; Zhou, M. F. Chin. Chem. Lett. 2003, 14, 1246.
[22] Liu, W.; Xu, Q.; Chen, B.; Ma, Y. Synth. Commun. 2002, 32, 171.
[23] Salisova, M.; Puciova, M.; Postnov, U. N.; Toma, S. Chem. Papers 1990, 44, 201.
[24] Villemin, D.; Martin, B.; Puciova, M.; Toma, S. J. Organomet. Chem. 1994, 484, 27.
/
| 〈 |
|
〉 |