

含氨基蒽醌信号单元的咔唑磺胺类阴离子受体的合成及识别性能研究
收稿日期: 2015-12-11
修回日期: 2016-01-21
网络出版日期: 2016-02-02
基金资助
国家自然科学基金(No.21161005)资助项目.
Synthesis and Recognition Properties of Carbazole Sulfonamide-Based Anion Receptor Containing the Aminoanthraquinone Signalling Units
Received date: 2015-12-11
Revised date: 2016-01-21
Online published: 2016-02-02
Supported by
Project supported by the National Natural Science Foundation of China (No. 21161005).
谭赞, 杜欢, 吕新阳, 鲍小平 . 含氨基蒽醌信号单元的咔唑磺胺类阴离子受体的合成及识别性能研究[J]. 有机化学, 2016 , 36(6) : 1389 -1394 . DOI: 10.6023/cjoc201512016
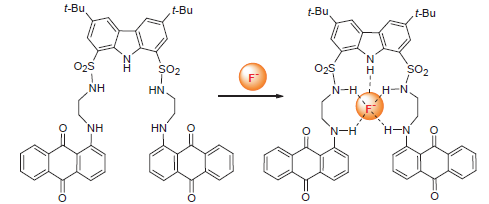
A novel carbazole sulfonamide-based anion receptor (L) containing the aminoanthraquinone signalling units was designed and synthesized, which was characterized by 1H NMR, 13C NMR, MS, IR and elemental analysis. The obtained results from UV-Vis and fluorescence titrations indicated that receptor L realized a highly selective recognition of F- via the formation of a 1:1 H-bonding complex. Moreover, the presence of F- induced a significant fluorescent quenching and a color change (from red to purple-red) of dimethyl sulfoxide (DMSO) solution of L, suggesting its potential as fluorescent and colorimetric sensors for the detection of F-.
[1] Schmidtchen, F. P.; Berger, M. Chem. Rev. 1997, 97, 1609.
[2] Wenzel, M.; Hiscock, J. R.; Gale, P. A. Chem. Soc. Rev. 2012, 41, 480.
[3] Miao, R.; Zheng, Q.-Y.; Chen, C.-F.; Huang, Z.-T. Tetrahedron Lett. 2005, 46, 2155.
[4] Chen, Q.-Y.; Chen, C.-F. Tetrahedron Lett. 2004, 45, 6493.
[5] Ayoob, S.; Gupta, A. K. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433.
[6] Zhou, Y.; Zhang, J. F.; Yoon, J. Chem. Rev. 2014, 114, 5511.
[7] Zhang, F.; Tan, Z.; Yan, B.-R.; Pan, D.-W.; Bao, X.-P. Chin. J. Org. Chem. 2014, 34, 2499 (in Chinese). (张峰, 谭赞, 闫柏任, 潘顶伍, 鲍小平, 有机化学, 2014, 34, 2499.)
[8] Sakai, R.; Barasa, E. B.; Sakai, N.; Sato, S.-I.; Satoh, T.; Kakuchi, T. Macromolecules 2012, 45, 8221.
[9] Chen, C.-F.; Chen, Q.-Y. Tetrahedron Lett. 2004, 45, 3957.
[10] Lu, H.; Xu, W.; Zhang, D.; Chen, C.; Zhu, D. Org. Lett. 2005, 7, 4629.
[11] Jung, H. S.; Kim, H. J.; Vicens, J.; Kim, J. S. Tetrahedron Lett. 2009, 50, 983.
[12] Liu, Y.; Zhang, F.; Zou, L.; Jian, J.; Bao, X. Chin. J. Org. Chem. 2013, 33, 2485 (in Chinese). (刘勇, 张峰, 邹林波, 蹇军友, 鲍小平, 有机化学, 2013, 33, 2485.)
[13] Jian, J.-Y.; Yan, B.-R.; Pan, D.-W.; Tan, Z.; Lv, X.-Y.; Du, H.; Bao, X.-P. Chin. J. Org. Chem. 2015, 35, 1069 (in Chinese). (蹇军友, 闫柏任, 潘顶伍, 谭赞, 吕新阳, 杜欢, 鲍小平, 有机化学, 2015, 35, 1069.)
[14] Chen, J.-J.; Liu, C.-X.; Zhang, J.-L.; Ding, W.; Zhou, M.; Wu, F.-H. Chem. Commun. 2013, 49, 10814.
[15] De Silva, A. P.; Gunaratne, H. Q. N.; Gunnlaugsson, T.; Huxley, A. J. M.; McCoy, C. P.; Rademacher, J. T.; Rice, T. E. Chem. Rev. 1997, 97, 1515.
[16] Valeur, B.; Pouget, J.; Bourson, J.; Kaschke, M.; Ernsting, N. P. J. Phys. Chem. 1992, 96, 6545.
[17] Bourson, J.; Pouget, J.; Valeur, B. J. Phys. Chem. 1993, 97, 4552.
[18] Liu, S.-Y.; Fang, L.; He, Y.-B.; Chan, W.-H.; Yeung, K.-T.; Cheng, Y.-K.; Yang, R.-H. Org. Lett. 2005, 7, 5825.
[19] Zhou, Y.-H.; Zheng, P.-C.; Bao, X.-P. Supramol. Chem. 2014, 26, 761. Kowalczyk, A.; Nowicka, A. M.; Jurczakowski, R.; Niedzial- kowski, P.; Ossowski, T.; Stojek, Z. Electroanalysis 2010, 22, 49.
/
| 〈 |
|
〉 |