

木质素氧化降解研究进展
收稿日期: 2015-11-25
修回日期: 2016-01-28
网络出版日期: 2016-02-01
基金资助
上海市大学生创新创业重点(No.PE2014032)、上海市科委地方院校能力建设(No.15120503700)资助项目.
Research Progress of Lignin Oxidative Degradation
Received date: 2015-11-25
Revised date: 2016-01-28
Online published: 2016-02-01
Supported by
Project supported by the Key Projects of Innovation and Entrepreneurship of College Students in Shanghai (No. PE2014032), and the Capacity-building Projects in Shanghai Local Universities (No. 15120503700).
张海峰 , 杨军艳 , 吴建新 , 毛海舫 , 孙小玲 . 木质素氧化降解研究进展[J]. 有机化学, 2016 , 36(6) : 1266 -1286 . DOI: 10.6023/cjoc201511049
Lignin is the second most abundant natural polymer. Oxidative degradation of lignin polymer is a very promising approach in lignin valorisation, which offers the possibility to provide highly functional monomer and oligomer products in the chemical and pharmaceutical industries instead of fossil fuels used as the starting materials of the process of other valorisation. This paper focuses on the oxidative modification methods of lignin and its model compounds, including biocatalysis, biomimetic catalysis, organometallic catalysis, electrochemistry catalytic oxidation and several other oxidation methods, and a brief discussion of the reaction mechanism in the process of oxidative degradation.
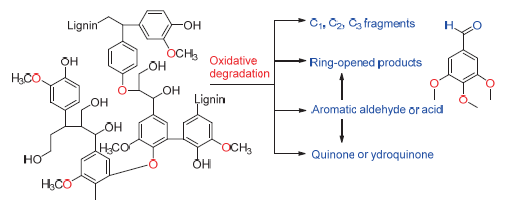
Key words: lignin; lignin valorisation; catalytic oxidation; degradation
[1] Dale, B. E.; Ong, R. G. Biotechnol. Prog. 2012, 28, 893.
[2] Stern, D. I. Ann. N. Y. Acad. Sci. 2011, 1219, 26.
[3] Bentley, R. W. Energy Policy 2002, 30, 189.
[4] Chang, C. C. Appl. Energy 2010, 87, 3533.
[5] Tverberg, G. E. Energy 2012, 37, 27.
[6] Zhang, N.; Lior, N.; Jin, H. Energy 2011, 36, 3639.
[7] Srirangan, K.; Akawi, L.; Moo-Young, M. Appl. Energy 2012, 100, 172.
[8] Lu, Y.; Wei, X.-Y.; Zong, Z.-M. Prog. Chem. 2013, 25, 838 (in Chinese). (路瑶,魏贤勇,宗志敏,化学进展, 2013, 25, 838.)
[9] Jiang,T.-D. Lignin, Chemical Industry Press, Beijing, 2008, p. 30 (in Chinese). (蒋挺大, 木质素, 化学工业出版社, 北京, 2008.)
[10] Crestini, C.; Crucianelli, M.; Orlandi, M. Catal. Today 2010, 156, 8 .
[11] Mu, Y.-B.; Wang, C.-P.; Zhao, L.-W.; Chu, F.-X. Chem. Ind. For. Prod. 2009, 29, 38 (in Chinese). (穆有炳, 王春鹏, 赵临五, 储富祥, 林产化学与工业, 2009, 29, 38.)
[12] El Mansouri, N. E.; Yuan, Q. L.; Huang, F. R. BioResources 2011, 6, 2492.
[13] Yue, X. P.; Chen, F. G.; Zhou, X. S. BioResources 2011, 6, 2022.
[14] Mohamad Ibrahim, M. N.; Ahmed-Haras, M. R.; Sipaut, C. S. Carbohydr. Polym. 2010, 80, 1102.
[15] Zhang, X. H.; Zhang, Q.; Wang, T. J.; Ma L. L. Bioresource Technol. 2013, 134, 73.
[16] Lange, H.; Decina, S.; Crestini, C. Eur. Poly. J. 2013, 49, 1151.
[17] Sun, R.; Lawther, J. M.; Banks, W. B. Ind. Crops Prod. 1995, 4, 241.
[18] Shi, L. L.; Yu, H. B.; Dong, T. B.; Kong W. Process Biochem. 2014, 49, 1097.
[19] Galli, C.; Gentili, P.; Acunzo, F. New J. Chem. 2006, 30, 583.
[20] Crestini, C.; D'Annibale, A.; Sermanni, G. G. Bioorg. Med. Chem. 2000, 8, 433.
[21] Crestini, C.; Saladino, R.; Tagliatesta, P. Bioorg. Med. Chem. 1999, 7, 1897.
[22] Zhu, W.; Ford, W. T. J. Mol. Catal. 1993, 78, 367.
[23] Gouvêa, C. A. K.; Wypych, F.; Moraes, S. G. Chemosphere 2000, 40, 427.
[24] Xie, Y. -M.; Hu, Z. -J.; Wu, H. Chem. Ind. For. Prod. 2004, 24, 1 (in Chinese). (谢益民,胡周建, 伍红, 林产化学与工业, 2004, 24, 1.)
[25] Biannic, B.; Bozell, J. Org. Lett. 2013, 15, 2730.
[26] Sedai, B.; Diaz-Urrutia, C.; Baker, R. T. ACS Catal. 2013, 3, 3111.
[27] Crestini, C.; Pro, P.; Neri, V. Bioorg. Med. Chem. 2005, 13, 2569.
[28] Vladimir, A. G.; Craig, L. H.; Ira, A. W. Oxidative Delignification Chemistry, Montereal, 2001, P. 297.
[29] Zhu, H. B.; Wang, L.; Chen, Y. M. RSC Adv. 2014, 4, 29917.
[30] Cui X. M.; Liang J. D.; Wang D. Q. J. Chem. Technol. Biotechnol. 2015, 90, 747.
[31] Rahimi, A.; Azarpira, A.; Kim H. J. Am. Chem. Soc. 2013, 135, 6415.
[32] Badamali, S. K.; Luque, R.; Clark, J. H. Catal. Commun. 2013, 31, 1.
[33] Nie, S.; Liu, X.; Wu, Z. Chem. Eng. J. 2014, 241, 410.
[34] Ferm, R.; Kringsta, K. P.; Cowling, E. B. Sven. Papperstidn. 1972, 75, 859.
[35] Wariishi, H.; Akileswaran, L.; Gold, M. H. Biochemistry 1988, 27, 5365.
[36] Wariishi, H.; Valli, K.; Gold, M. H. J. Biol. Chem. 1992, 267, 23688.
[37] Wariishi, H.; Valli, K.; Gold, M. H. Biochemistry 1989, 28, 6017.
[38] Tuor, U.; Wariishi, H.; Schoemaker, H. E.; Gold, M. H. Biochemistry 1992, 31, 4986.
[39] D'Annibale, A.; Crestini, C.; Mattia, E. D.; Sermanni G. G. J. Biotechnol. 1996, 48, 231.
[40] Saliu, F.; Tolppa, E.; Zoia, L.; Orlandi, M. Tetrahedron Lett. 2011, 52, 3856.
[41] Claudia ,C.; Raffaella, P.; Raffaele, S. Appl. Catal. A-Gen. 2010, 372, 115.
[42] Lundquist, K.; Kristersson, P. Biochem. J. 1985, 229, 277.
[43] Call, H. P.; Mücke, I. J. Biotechnol. 1997, 53, 163.
[44] Kirk, T. K.; Chang, H. M.; Butterworth-Heinemann, B. Massachusetts. 1990, 666, 99.
[45] Higuchi, T. Wood Sci. Technol. 1990, 24, 23.
[46] Shleev, S.; Christenson, A.; Serezhenkov, V. Biochem. J. 2005, 385, 745.
[47] Messerschmidt, A.; Huber, R. Eur. J. Biochem. 1990, 187, 341.
[48] Majcherczyk, A.; Johannes, C.; Hüttermann, A. Appl. Microbiol. Biot. 1999, 51, 267.
[49] Crestini, C.; Jurasek, L.; Argyropoulos, D. S. Chem.-Eur. J. 2003, 9, 5371.
[50] Rodríguez, C. S.; Osma, J. F.; Saravia, V. Appl. Catal. A-Gen. 2007, 329, 156.
[51] Krajewska, B. Enzyme Microb. Technol. 2004, 35, 126.
[52] Cho, Y. K.; Bailey, J. E. Biotechnol. Bioeng. 1979, 21, 461.
[53] Abadulla, E.; Tzanov, T.; Costa, S. Appl. Environ. Microb. 2000, 66, 3357.
[54] Ryan, S.; Schnitzhofer, W.; Tzanov, T. Enzyme Microb. Technol. 2003, 33, 766.
[55] Kandelbauer, A.; Maute, O.; Kessler, R. W. Biotechnol. Bioeng. 2004, 87, 552.
[56] Osma, J. F.; Toca-Herrera, J. L.; Rodríguez-Couto, S. Bioresource Technol. 2010, 101, 8509.
[57] Held, C.; Kandelbauer, A.; Schroeder, M. Environ. Chem. Lett. 2005, 3, 74.
[58] Decher, G.; Hong, J. D.; Schmitt, J. Thin Solid Films 1992, 210, 831.
[59] Peyratout, C. S.; Dähne, L. Angew. Chem., Int. Ed. 2004, 43, 3762.
[60] Rochefort, D.; Kouisni, L.; Gendron, K. J. Electroanal. Chem. 2008, 617, 53.
[61] Hébert, M.; Rochefort, D. ECS Trans. 2008, 16, 85.
[62] Di, S. M.; Maturo, C.; De, A. E. Catal. Today 2003, 79, 333.
[63] Crestini, C.; Perazzini, R.; Saladino, R. Appl. Catal. A-Gen. 2010, 372, 115.
[64] Guazzaroni, M.; Crestini, C.; Saladino, R. Bioorg. Med. Chem. 2012, 20, 157.
[65] Perazzini, R.; Saladino, R.; Guazzaroni, M. Bioorg. Med. Chem. 2011, 19, 440.
[66] Crestini, C.; Melone, F.; Saladino, R. Bioorg. Med. Chem. 2011, 19, 5071.
[67] Tien, M.; Kirk, T. K. Science 1983, 221, 661.
[68] Song, R.; Sorokin, A.; Bernadou, J. J. Org. Chem. 1997, 62, 673.
[69] Venkatasubbaiah, K.; Zhu, X.; Kays, E. ACS Catal. 2011, 1, 489.
[70] Baker, J. H. F. M. S. Thesis, Eastern Kentucky University, Kentucky, 2012.
[71] Jiang, Q.; Sheng, W.; Guo, X. J. Mol. Catal. A-Chem. 2013, 373, 121.
[72] Crestini, C.; Saladino, R.; Tagliatesta, P. Bioorg. Med. Chem. 1999, 7, 1897.
[73] Crestini, C.; Pastorini, A.; Tagliatesta, P. Eur. J. Inorg. Chem. 2004, 2004, 4477.
[74] Zucca, P.; Sollai, F.; Garau, A. J. Mol. Catal. A 2009, 306, 89.
[75] Kumar, A.; Jain, N.; Chauhan, S. M. S. Synlett 2007, 411.
[76] Crestini, C.; Pastorini, A.; Tagliatesta, P. J. Mol. Catal. A 2004, 208, 195.
[77] Zucca, P.; Mocci, G.; Rescigno, A. J. Mol. Catal. A 2007, 278, 220.
[78] Barros, V. P.; Faria, A. L.; MacLeod, T. C. O. Int. Biodeterior Biodegrad. 2008, 61, 337.
[79] Pattou, D.; Labat, G.; Defrance, S. B. Soc. Chim. Fr. 1994, 131, 78.
[80] Shimada, M.; Habe, T.; Higuchi, T. Holzforschung 1987, 41, 277.
[81] Cui, F.; Dolphin, D. Bioorg. Med. Chem. Lett. 1995, 3, 471.
[82] Barbat, A.; Gloaguen, V.; Sol, V. Bioresource Technol. 2010, 101, 6538.
[83] Meunier, B.; Sorokin, A. Acc. Chem. Res. 1997, 30, 470.
[84] Kawai, S.; Ohashi, H. Phys. Technol. Wood 1993, 47, 97.
[85] Barton, D. H. R.; Delanghe, N. C.; Patin, H. Tetrahedron 1997, 53, 16017.
[86] Walker, C. C.; Dinus, R. J.; McDonough, T. J. Holzforschung 1999, 53,181.
[87] Watanabe, T.; Koller, K.; Messner, K. J. Biotechnol. 1998, 62, 221.
[88] Mirkhani, V.; Moghadam, M.; Tangestaninejad, S. Catal. Commun. 2008, 9, 219.
[89] Badamali, S. K.; Luque, R.; Clark, J. H. Catal. Commun. 2011, 12, 993.
[90] Gupta, K. C.; Sutar, A. K. J. Mol. Catal. A 2007, 272, 64.
[91] Cedeno, D.; Bozell, J. J. Tetrahedron Lett. 2012, 53, 2380. .
[92] Kogami, Y.; Nakajima, T. Ikeno, T.; Yamada, T. Synthesis 2004, 1947.
[93] Park, J.; Lang, K.; Abboud, K. A. J. Am. Chem. Soc. 2008, 130, 16484.
[94] Guo, X. F.; Kim, Y. S.; Kim, G. J. Top. Catal. 2009, 52, 153.
[95] Bozell, J.; Elder, T. J. Green Chem. 2014, 16, 3635.
[96] Zakzeski, J.; Jongerius, A. L.; Weckhuysen, B. M. Green Chem. 2010, 12, 1225.
[97] Watanabe, E.; Kaiho, A.; Kusama, H. J. Am. Chem. Soc. 2013, 135, 11744.
[98] Sonar, S.; Ambrose, K.; Hendsbee, A. D. Can. J. Chem. 2011, 90, 60.
[99] Zhou, X. -F. J. Appl. Polym. Sci. 2014, 131, 9594.
[100] Bernini, R.; Mincione, E.; Cortese, M. Tetrahedron Lett. 2001, 42, 5401.
[101] Kühn, F. E.; Scherbaum, A.; Herrmann, W. A. J. Organomet. Chem. 2004, 689, 4149.
[102] Zhu, Z.; Espenson, J. H. J. Org. Chem. 1995, 60, 7728.
[103] Jacob, J.; Espenson, J. H. Inorg. Chim. Acta 1998, 270, 55.
[104] Herrmann, W. A.; Fischer, R. W.; Scherer, W. Angew. Chem., Int. Ed. 1993, 32, 1157.
[105] Adam, W.; Herrmann, W. A.; Saha-Möller, C. R. J. Mol. Catal. A 1995, 97, 15.
[106] Amorati, R.; Pedulli, G. F.; Valgimigli, L.; Attanasi, O. A.; Filippone, P.; Fiorucci, C.; Saladino, R. J. Chem. Soc., Perkin Trans. 2 2001, 2142.
[107] Saladino, R.; Gualandi, G.; Farina, A. Curr. Med. Chem. 2008, 15, 1500.
[108] Saladino, R.; Neri, V.; Mincione, E. Marini,S.; Coletta, M.; Fiorucci, C.; Filippone, P. J. Chem. Soc., Perkin Trans. 1 2000, 581.
[109] Saladino, R.; Mincione, E.; Attanasi, O. A. Pure Appl. Chem. 2003, 75, 265.
[110] Saladino, R.; Neri, V.; Mincione, E. Tetrahedron 2002, 58, 8493.
[111] Crestini, C.; Caponi, M. C. Argyropoulos, D. S. Bioorg. Med. Chem. 2006, 14, 5292.
[112] Saladino, R.; Neri, V.; Pelliccia, A. R. J. Org. Chem. 2002, 67, 1323.
[113] Bujanovic, B.; Ralph, S.; Reiner, R. Mater 2010, 3, 1888.
[114] Lv, H.; Geletii, Y. V.; Zhao, C. Chem. Soc. Rev. 2012, 41, 7572.
[115] Gaspar, A.; Evtuguin, D. V.; Pascoal, N. C. Appl. Catal. A 2003, 239, 157.
[116] Zhao, Y.; Xu, Q.; Pan, T. Appl. Catal. A 2013, 467, 504.
[117] Yokoyama, T.; Chang, H.; Reiner, R. S. Holzforschung 2004, 58, 116.
[118] Voitl, T.; Rudolf, . R. P. V. ChemSusChem 2008, 1, 763.
[119] Weinstock, I. A.; Barbuzzi, E. M. G.; Wemple, M. W. Nature 2001, 414, 191.
[120] Hoover, J. M.; Ryland, B. L.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 2357.
[121] Hoover, J. M.; Stahl, S. S. J. Am. Chem. Soc. 2011, 133, 16901.
[122] Ansari, I. A.; Gree, R. Org. Lett. 2002, 4, 1507.
[123] Dijksman, A.; Marino-Gonzalez, A.; Mairata, I. P. A. J. Am. Chem. Soc. 2001, 123, 6826.
[124] Zhao, M.; Li, J.; Mano, E. J. Am. Chem. Soc. 1999, 64, 2564.
[125] Karimi, B.; Farhangi, E. Chem.-Eur. J. 2011, 17, 6056.
[126] Nooy, A. E. J. D.; Besemer, A. C.; Bekkum, H. V. Carbohydr. Res. 1995, 269, 89.
[127] Kato, Y.; Matsuo, R.; Isogai, A. Carbohydr. Polym. 2003, 51, 69.
[128] Jiang, B.; Drouet, E.; Milas, M. Carbohydr. Res. 2000, 327, 455.
[129] Fraschini, C.; Vignon, M. R. Carbohydr. Res. 2000, 328, 585.
[130] Saito, T.; Nishiyama, Y. ; Putaux, J. L. Biomacromolecules 2006, 7, 1687.
[131] Saito, T.; Isogai, A. Biomacromolecules 2004, 5, 1983.
[132] Sedai, B.; Diaz-Urrutia, C.; Baker, R. T. C. ACS Catal. 2011, 1, 794.
[133] Sedai, B.; Baker, R. T. Adv. Synth. Catal. 2014, 356, 3563.
[134] Omori, S.; Dence, C. W. Wood Sci. Technol. 1981, 15, 67.
[135] Sawaki, Y.; Foote, C. S. J. Am. Chem. Soc. 1983, 105, 5035.
[136] Lanzalunga, O.; Bietti, M. J. Photochem. Photobiol. B 2000, 56, 85.
[137] Kovalenko, E. I.; Popova, O. V.; Aleksandrov, A. A. Russ. J. Electrochem. 2000, 36, 706.
[138] Parpot, P.; Bettencourt, A. P.; Carvalho, A. M. J. Appl. Electrochem. 2000, 30, 727.
[139] Pelegrini, R.; Reyes, J.; Duran, N. J. Appl. Electrochem. 2000, 30, 953.
[140] Lu, Z.; Tu, B.; Chen, F. J. Wood Chem. Technol. 2003, 23, 261.
[141] Tolba, R.; Tian, M.; Wen, J. J. Electroanal. Chem. 2010, 649, 9.
[142] Tian, M.; Wen, J.; MacDonald, D. Electrochem. Commun. 2010, 12, 527.
[143] Pan, K.; Tian, M. ; Jiang, Z. H. Electrochim. Acta. 2012, 60, 147.
[144] Brandt, A.; Hallett, J. P.; Leak, D. J. Green Chem. 2010, 12, 672.
[145] Liu, S.; Shi, Z.; Li, L. RSC Adv. 2013, 3, 5789.
[146] Reichert, E.; Wintringer, R.; Volmer, D. A. Phys. Chem. Chem. Phys. 2012, 14, 5214.
[147] Shao, D.; Liang, J.; Cui, X. Chem. Eng. J. 2014, 244, 288.
[148] Cui, X. M, Liang, J. D, Wang, D. Q. J. Chem. Technol. Biotechnol. 2015, 90, 747.
[149] Voort, P. V. D.; Vercaemst, C.; Schaubroeck, D. Phys. Chem. Chem. Phys. 2008, 10, 347.
[150] Stein, A.; Melde, B. J.; Schroden, R. C. Adv. Mater. 2000, 12, 1403.
[151] Itoh, A.; Kodama, T.; Masaki, Y. Chem. Pharm. Bull. 2006, 54, 1571.
[152] Badamali, S. K.; Clark, J. H.; Breeden, S. W. Catal. Commun. 2008, 9, 2168.
[153] Beck, J. S.; Vartuli, J. C.; Roth, W. J. J. Am. Chem. Soc. 1992, 114, 10834.
[154] Badamali, S. K.; Luque, R.; Clark, J. H. Catal. Commun. 2009, 10, 1010.
[155] Sun, Y.; Zhang, J.-P.; Yang, G. Spectrosc. Spect. Anal. 2007, 27, 1551 (in Chinese). (孙勇, 张金平,杨刚, 光谱学与光谱分析, 2007, 27, 1551.)
[156] Hoareau, W.; Trindade, W. G.; Siegmund, B. Polym. Degrad. Stabil. 2004, 86, 567.
[157] Nie, S.; Liu, X.; Wu, Z. Chem. Eng. J. 2014, 241, 410.
[158] Svenson, D. R.; Kadla, J. F.; Chang, H. Ind. Eng. Chem. Res. 2002, 41, 5927.
[159] Das, L.; Kolar, P.; Sharma-Shivappa, R. Biofuels 2012, 3, 155.
[160] Kobayakawa, K.; Sato, Y.; Nakamura, S. B. Chem. Soc. Jpn. 1989, 62, 3433.
[161] Gouvêa, C. A. K.; Wypych, F.; Moraes, S. G. Chemosphere 2000, 40, 427.
[162] Machado, A. E. H. Furuyama, A. M.; Falone, S. Z. Chemosphere 2000, 40, 115.
[163] Kansal, S. K.; Singh, M. Sud, D. J. Hazard. Mater. 2008, 153, 412.
[164] Ma, Y. S.; Chang, C. N.; Chiang, Y. P. Chemosphere 2008, 71, 998.
[165] Portjanskaja, E.; Stepanova, K.; Klauson, D. Catal. Today 2009, 144, 26.
[166] Rangel, R.; Mercado, G. J.; Bartolo-Pérez, P. Sci. Adv. Mater. 2012, 4, 573.
[167] Li, R.-X. Green Solvent—the Synthesis and Application of Ionic Liquids, Chemical Industry Press, Beijing, 2004 (in Chinese). (李汝雄, 绿色溶剂——离子液体的合成与应用, 化学工业出版社北京, 2004.)
[168] Sheldon, R. A.; Lau, R. M.; Sorgeddrager, M. J.; Rantwijk, F. V.; Seddon, K. R. Green Chem. 2002, 4, 147.
[169] Sang, H. L.; Thomas, V. D.; Robert, J. L.; Jonathan, S. D. Biotechnol. Bioeng. 2009, 102, 1368.
[170] Fort, D. A.; Remsing, R. C.; Swatloski ,R. P.; Rogers, R. D. Green Chem. 2007, 9, 63.
[171] Michael, Z.; Daniela B.; Matthias F.; Jochen, B.; Antje, C. S. Bioresour. Technol. 2009, 100, 2580.
[172] Sun, N.; Rahman, M.; Qin,Y.; Mirela, L. M.; Robin, D. R. Green Chem. 2009, 11, 646.
[173] Pu, Y. Q.; Nan, J.; Arthur, J. R. Wood Chem. Technol. 2007, 27, 23.
[174] Tan, S. S.Y.; MacFarlane, D. R.; Edye, L. A.; Pringle, J. M. Green Chem. 2009, 11, 339.
[175] Jürgen, V.; Tina, E.; Claudia, H.; Ulrich, S. S. Green Chem. 2009, 11, 417.
[176] Magorzata, E. Z.; Bogel-Lukasik, E. Energy Fuels 2010, 24, 737.
[177] Nan, J.; Arthur, J. R. J. Org. Chem. 2007, 72, 7030.
[178] Stärk, K.; Taccardi, N.; Bösmann, A. ChemSusChem 2010, 3, 719.
[179] Sun, X. L.; Yang, J.; Zhang, H.; Shen, Y.; Bi, Y.; Miao, H.; Mao, H. CN 105037116A 2015 [Chem. Abstr. 2015, 163, 702069].
/
| 〈 |
|
〉 |