

Lewis/Brønsted酸催化炔丙醇在亲核取代反应中的研究进展
收稿日期: 2015-12-02
修回日期: 2015-12-30
网络出版日期: 2016-02-01
基金资助
国家自然科学青年基金(No.21302096)、江苏省自然科学青年基金(Nos.BK20130962,BK20130952)及江苏高校优势学科建设工程(PAPD)资助项目.
Progress of the Research on the Lewis/Brønsted Acid-Catalyzed Nucleophilic Substitution of Propargyl Alcohols
Received date: 2015-12-02
Revised date: 2015-12-30
Online published: 2016-02-01
Supported by
Project supported by the Young National Natural Science Foundation of China (No. 21302096), the Young Natural Science Foundation of Jiangsu Province (Nos. BK20130962, BK20130952) and the Project Fund from the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
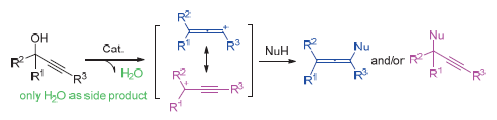
Lewis/Brønsted酸催化炔丙醇的亲核取代反应在有机合成化学中具有十分重要的地位, 炔丙醇可被转化为种类多样的无环、碳环以及杂环等重要的合成砌块. 直接以炔丙醇炔丙基化的方法的优点在于只产生副产物水, 而传统的Nicholas炔丙基化合成法会产生大量的废弃物, 相比之下前者是一种绿色的合成方法. 综述了炔丙醇在路易斯酸或者布朗斯特酸催化下与不同的亲核试剂(NuH=C, N, O, S, I)发生分子内和分子间亲核取代反应构筑碳-碳、碳-杂键的最新研究进展. 最后就炔丙基化研究及应用中存在的问题和难点对其前景进行了展望.
关键词: 炔丙醇; 亲核取代; Lewis/Brønsted酸催化
张小祥, 吕昌, 李萍, 付博, 姚薇薇 . Lewis/Brønsted酸催化炔丙醇在亲核取代反应中的研究进展[J]. 有机化学, 2016 , 36(6) : 1287 -1298 . DOI: 10.6023/cjoc201512003
Lewis/Brønsted acid-catalyzed nucleophilic substitution of propargylic alcohols is very important in organic synthetic chemistry, which could be transformed into a variety of acyclic, carbocyclic and heterocyclic synthetic building blocks. A drawback of the traditional propargylation of Nicholas reaction is the generation of waste products resulting from displacement of the leaving group on treating with a catalyst and/or nucleophile. Therefore, the direct acid-catalyzed propargylation of propargylic alcohols is considered as a green method, which generated water as the only side product. In this review, the latest research progress on the Lewis and Brønsted acids catalyzed intermolecular and intramolecular propargylation of a variety of nucleophiles (NuH=C, N, O, S, I) with propargylic alcohols is presented. Finally, the problems and difficulties in research and application of propargylation of propargylic alcohols are discussed and then prospective is provided.

[1] (a) Ayers, B. J.; Chan, P. W. H. Synlett 2015, 26, 1305.
(b) Zhu, Y.; Sun, L.; Lu, P.; Wang, Y. ACS Catal. 2014, 4, 1911.
(c) Patil, N. T.; Kavthe, R. D.; Shinde, V. S. Tetrahedron 2012, 68, 8079.
(d) Zhang, L.; Fang, G.; Kumar, R. K.; Bi, X. Synthesis 2015, 47, 2317.
(e) Yang, Z.; Kumar, R. K.; Liao, P.; Liu, Z.; Li, X.; Bi, X. Chem. Commun. 2016, 52, 5936.
(f) Patil, N. T.; Kavthe, R. D.; Shinde, V. S. Tetrahedron 2012, 68, 8079.
(g) Shu, X.-Z.; Shu, D.; Schienebeck, C. M.; Tang, W. Chem. Soc. Rev. 2012, 41, 7698.
(h) Leyva-Pérez, A.; Corma, A. Angew. Chem., Int. Ed. 2012, 51, 614.
(i) Hao, L.; Zhan, Z.-P. Curr. Org. Chem. 2011, 15, 1625.
(j) Michelet, V.; Toullec, P. Y.; Genêt, J.-P. Angew. Chem., Int. Ed. 2008, 47, 4268.
(k) Patil, N. T.; Yamamoto, Y. Chem. Rev. 2008, 108, 3395.
(l) Yamamoto, Y. J. Org. Chem. 2007, 72, 7817.
[2] (a) Zhang, X.; Sun, X.; Tan, J.; Fan, H.; Rao, W. Chin. J. Org. Chem. 2015, 35, 2049 (in Chinese). (张小祥, 孙小萍, 谈继淮, 樊辉, 饶卫东, 有机化学, 2015, 35, 2049.)
(b) Zhang, X.; Sun, X.; Rao, W. Chin. J. Org. Chem. 2015, 35, 1500 (in Chinese). (张小祥, 孙小萍, 饶卫东, 有机化学, 2015, 35, 1500.)
(c) Zhang, X.; Teo, J. W.; Ma, D.-L.; Leung, C.-H.; Chan, P. W. H. Tetrahedron Lett. 2014, 55, 6703.
(d) Zhang, X.; Teo, W. T.; Rao, W.; Ma, D.-L.; Leung, C.-H.; Chan, P. W. H. Tetrahedron Lett. 2014, 55, 3881.
(e) Zhang, X.; Teo, W. T.; Chan, P. W. H. J. Organomet. Chem. 2011, 696, 331.
(f) Zhang, X.; Teo, W. T.; Sally, Chan, P. W. H. J. Org. Chem. 2010, 75, 6290.
(g) Zhang, X.; Teo, W. T.; Chan, P. W. H. Org. Lett. 2009, 11, 4990.
(h) Kothandaraman, P.; Rao, W.; Zhang, X.; Chan, P. W. H. Tetrahedron 2009, 65, 1833.
(i) Rao, W.; Zhang, X.; Sze, E. M. L.; Chan, P. W. H. J. Org. Chem. 2009, 74, 1740.
(j) Zhang, X.; Rao, W.; Sally, Chan, P. W. H. Org. Biomol. Chem. 2009, 7, 4186.
(k) Zhang, X.; Rao, W.; Chan, P. W. H. Synlett 2008, 2204.
[3] (a) Caffyn, A. J. M.; Nicholas, K. M. In Comprehensive Organometallic Chemistry II, Vol. 12, Eds.: Abel, E. W.; Stone, F. G. A.; Wilkinson, J., Pergamon, Oxford, 1995, Chapter 7.1.
(b) Nicholas, K. M.; Pettit, R. J. Organomet. Chem. 1972, 44, 21.
(c) Nicholas, K. M. Acc. Chem. Res. 1987, 20, 207.
(d) Green, J. R. Curr. Org. Chem. 2001, 5, 809.
(e) Teobald, B. J. Tetrahedron 2002, 58, 4133.
(f) Kuhn, O.; Rau, D.; Maayr, H. J. Am. Chem. Soc. 1998, 120, 900. (g) Müller, T. J. J. Eur. J. Org. Chem. 2001, 2021.
(h) Nishibayashi, Y.; Uemura, S. Curr. Org. Chem. 2006, 10, 135.
[4] Georgy, M.; Boucard, V.; Campagne, J. M. J. Am. Chem. Soc. 2005, 127, 14180.
[5] Zhan, Z.; Wang, W.; Yang, R.; Yu, J.; Li, J.; Liu, H. Chem. Commun. 2006, 3352.
[6] Zhan, Z.; Liu, J.; Liu, H.; Cui, Y.; Yang, R.; Yang, W.; Li, J. J. Org. Chem. 2006, 71, 8298.
[7] Sanz, R.; Miguel, D.; Martinez, A.; Alvarez-Gutierrez, J. M.; Rodriguez, F. Org. Lett. 2007, 9, 727.
[8] Yoshimatsu, M.; Otani, T.; Matsuda, S.; Yamamoto, T.; Sawa, A. Org. Lett. 2008, 10, 4251.
[9] Sanz, R.; Miguel, D.; Martinez, A.; Gohain, M.; Garcia-Garcia, P.; Fernandez-Rodriguez, A. M.; Alvarez, E.; Rodriguez, F. Eur. J. Org. Chem. 2010, 36, 7027.
[10] Silveira, C. C.; Mendes, S. R.; Wolf, L.; Martins, G. M. Tetrahedron Lett. 2010, 51, 4560.
[11] Gohain, M.; Marais, C.; Bezuidenhoudt, B. C. B. Tetrahedron Lett. 2012, 53, 4704.
[12] Yadav, J. S.; Reddy, B. V. S.; Thrimurtulu, N.; Mallikarjuna Reddy, N.; Prasad, A. R. Tetrahedron Lett. 2008, 49, 2031.
[13] Wang, T.; Ma, R. D.; Liu, L.; Zhan, Z. P. Green Chem. 2010, 12, 1576.
[14] Huang, W.; Shen, Q.; Wang, J.; Zhou, X. J. Org. Chem. 2008, 73, 1586.
[15] Huang, W.; Zheng, P.; Zhang, Z.; Liu, R.; Chen, Z.; Zhou, X. J. Org. Chem. 2008, 73, 6845.
[16] Chatterjee, P. N.; Roy, S. J. Org. Chem. 2010, 75, 4413.
[17] Zhao, W.; Carreira, E. M. Org. Lett. 2003, 5, 4153.
[18] Yuan, F. Q.; Han, F. S. Adv. Synth. Catal. 2013, 355, 537.
[19] Zhang, L.; Zhu, Y. X.; Yin, G.; Lu, P.; Wang, Y. J. Org. Chem. 2012, 77, 9510.
[20] Hao, L.; Pan, Y.; Wang, T.; Lin, M.; Chen, L.; Zhan, Z. Adv. Synth. Catal. 2010, 352, 3215.
[21] Song, J.-N.; Fang, Z.; Liu, Y.; Li, R.; Xu, L.; Barry, B.-D.; Liu, Q.; Bi, X.; Liao, P. Synlett 2011, 2551.
[22] Li, Q.; Wang, Y.; Fang, Z.; Liao, P.; Barry, B.-D.; Che, G.; Bi, X. Synthesis 2013, 45, 609.
[23] Liu, Y.; Barry, B.-D.; Yu, H.; Liu, J.; Liao, P.; Bi, X. Org. Lett. 2013, 15, 2608.
[24] Ji, K.-G.; Shu, X.-Z.; Zhao, S.-C.; Zhu, H.-T.; Niu, Y.-N.; Liu, X. Y.; Liang, Y.-M. Org. Lett. 2009, 11, 3206.
[25] Haven, T.; Kubik, G.; Haubenreisser, S.; Niggemann, M. Angew. Chem., Int. Ed. 2013, 52, 4016.
[26] Shi, M.; Yao, L.-F. Chem.-Eur. J. 2008, 14, 8725.
[27] Yao, L.-F.; Wei, Y.; Shi, M. J. Org. Chem. 2009, 74, 9466.
[28] Huang, G. B.; Wang, X.; Pan, Y. M.; Wang, H. S.; Yao, G. Y.; Zhang, Y. J. Org. Chem. 2013, 78, 2742.
[29] Yin, G.; Zhu, Y.; Lu, P.; Wang, Y. J. Org. Chem. 2011, 76, 8922.
[30] (a) Yin, G.; Zhu, Y.; Wang, N.; Lu, P.; Wang, Y. Tetrahedron 2013, 69, 8353.
(b) Similar reference, see: Shao, Y.; Zhu, K.; Qin, Z.; Li, E.; Li, Y. J. Org. Chem. 2013, 78, 5731.
[31] Wang, S. Y.; Zhu, Y. X.; Wang, Y. G.; Lu, P. Org. Lett. 2009, 11, 2615.
[32] Zhu, Y.; Yin, G.; Hong, D.; Lu, P.; Wang, Y. Org. Lett. 2011, 13, 1024.
[33] Zhu, Y. X.; Wen, S.; Yin, G. W.; Hong, D.; Lu, P.; Wang, Y. G. Org. Lett. 2011, 13, 3553.
[34] Yin, G. W.; Zhu, Y. X.; Zhang, L.; Lu, P.; Wang, Y. G. Org. Lett. 2011, 13, 940.
[35] Mothe, S. R.; Kothandaraman, P.; Lauw, S. J. L.; Chin, S. M. W.; Chan, P. W. H. Chem.-Eur. J. 2012, 18, 6133.
[36] Mothe, S. R.; Novianti, M. L.; Ayers, B. J.; Chan, P. W. H. Org. Lett. 2014, 16, 4110.
[37] (a) Meyer, K. H.; Schuster, K. Ber. 1922, 55B, 819.
(b) Swaminathan, S.; Narayanan, K. V. Chem. Rev. 1971, 71, 429.
(c) Engel, D. A.; Dudley, G. B. Org. Biomol. Chem. 2009, 7, 4149.
[38] Aponick, A.; Li, C.-Y.; Palmes, J. A. Org. Lett. 2009, 11, 121.
[39] Wang, L.; Xie, X.; Liu, Y. Org. Lett. 2012, 14, 5848.
[40] Knight, D. W. WO 2006100479, 2006[Chem. Abstr. 2006, 145, 377330].
[41] Aponick, A.; Li, C.-Y.; Malinge, J.; Marques, E. F. Org. Lett. 2009, 11, 4624.
[42] Egi, M.; Azechi, K.; Akai, S. Org. Lett. 2009, 11, 5002.
[43] Chen, S.; Wang, J. J. Org. Chem. 2007, 72, 4993.
[44] Ji, K. G.; Zhu, H. T.; Yang, F.; Shu, X. Z.; Zhao, S. C.; Liu, X. Y. Shaukat, A.; Liang, Y. M. Chem.-Eur. J. 2010, 16, 6151.
[45] Zhu, H. T.; Ji, K. G.; Yang, F.; Wang, L. J.; Zhao, S. C.; Ali, S.; Liu, X. Y.; Liang, Y. M. Org. Lett. 2011, 13, 684.
[46] Yang, F.; Jin, T.; Bao, M.; Yamamoto, Y. Tetrahedron Lett. 2011, 52, 936.
[47] Yang, F.; Jin, T.; Bao, M.; Yamamoto, Y. Chem. Commun. 2011, 47, 4541.
[48] Choi, J.; Lee, G. H.; Kim, I. Synlett 2008, 1243.
/
| 〈 |
|
〉 |