

仲胺介入的丙炔酸甲酯与亚胺的环合反应
收稿日期: 2015-12-31
修回日期: 2016-01-20
网络出版日期: 2016-02-24
Secondary Amine-Mediated Cyclization of Methyl Propiolate with Imines
Received date: 2015-12-31
Revised date: 2016-01-20
Online published: 2016-02-24
马鸿飞 , 黄浩 , 宿江岭 , 钮长盛 , 吴正光 , 卜洪忠 , 李玉峰 . 仲胺介入的丙炔酸甲酯与亚胺的环合反应[J]. 有机化学, 2016 , 36(6) : 1335 -1340 . DOI: 10.6023/cjoc201512050
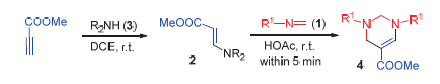
A facile method for the synthesis of tetrahydropyrimidines is described. Diethylamine was treated with methyl propiolate to provide Michael adduct (β-amino acrylate). In the presence of organic acid, the cyclization of imines with β-aminoacrylateoccurred at room temperature and completed within several minutes to give tetrahydropyrimidines with liberation of ammonium salt. Formaldimines show good toleration to the reaction.
Key words: methylpropiolate; imine; amine-mediated reaction; tetrahydropyrimidines
[1] Catherine, A.; Scott, J. J. Am. Chem. Soc. 2003, 125, 12394.
[2] Varinder, K.; Chrystel, L.; Franck S. J. Am. Chem. Soc. 2003, 125, 7596.
[3] Morris, M.; Michael, M.; Bernd, S.; Rainer, M. J. Am. Chem. Soc. 2007, 129, 7258
[4] Kerstin, R.; Rainer, M. Org. Lett. 2011, 13, 1878.
[5] De Lucca, G.; Liang, J.; De Lucca, I. J. Med. Chem. 1999, 42, 135.
[6] Dunbar, P.; Durant, G.; Rho, T.; Ojo, B.; Huzl, J.; Smith, A.; Sbeih, S.; Ngur, D.; Periyasamy, S.; Hoss, W.; Messer, W. J. Med. Chem. 1994, 37, 2774.
[7] Cao, H.; Wang, X.; Jiang, H.; Zhu, Q.; Zhang, M.; Liu, H. Chem. Eur. J. 2008, 14, 11623.
[8] Zhang, M.; Jiang, H.; Liu, H.; Zhu, Q. Org. Lett. 2007, 9, 4111.
[9] Zhu, Q.; Jiang, H.; Li, J.; Zhang, M.; Wang, X. Tetrahedron 2009, 65, 4604.
[10] Jin, T.; Zhao, Y.; Liu, L.; Li, T. Chin. J. Org. Chem.2006, 26, 975 (in Chinese). (靳通收, 赵莹, 刘利宾, 李同双, 有机化学, 2006, 26, 975.)
[11] Xue, N.; Chen, Y.; Lv, X.; Hu, Y. Chin. J. Org. Chem. 2008, 28, 325 (in Chinese). (薛娜, 陈也伟, 吕秀阳, 胡永洲, 有机化学, 2008, 28, 325.)
[12] Li, Y.; Shi, J.; Wu, Z.; Wang, X.; Wu, X.; Gu, J.; Bu, H.; Ma, H. Tetrahedron 2014, 70, 2472.
[13] Li, Y.; Wu, Z.; Shi, J.; Pan, Y.; Bu, H.; Ma, H.; Gu, J.; Huang, H.; Wang, Y.; Wu, L. Tetrahedron 2014, 70, 8971.
[14] Li, Y.; Wu, Z.; Shi, J.; Bu, H.; Gu, J.; Pan, Y. Tetrahedron 2014, 70, 3134.
[15] Sartori, G.; Bigi, F.; Maggi, R. Eur. J. Org. Chem. 2007, 72, 1315.
[16] Malkov, A.; Vrankova, K.; Stoncius, S. J. Org. Chem. 2009, 74, 5839.
[17] Jiang, Y.; Zhong, Y.; Lourdusamy, E.; Park, C. Chem. Commun. 2012, 48, 3133.
[18] Drev, M.; Groselj, U.; Mevec, S.; Pusavec, E.; Strekelj, J.; Golobic, A.; Dahmann, G.; Stanovnik, B.; Svete, J. Tetrahedron 2014, 70, 8267.
/
| 〈 |
|
〉 |