

8-氮杂双环[3.2.1]辛烷-3-异噁唑肟类衍生物的合成及其杀线虫活性
收稿日期: 2016-01-17
修回日期: 2016-02-29
网络出版日期: 2016-03-11
基金资助
国家自然科学基金(No.21572060)资助项目.
Synthesis and Nematicidal Activities of 8-Azabicyclo[3.2.1]-octane-3-isoxazole Oxime Derivatives
Received date: 2016-01-17
Revised date: 2016-02-29
Online published: 2016-03-11
Supported by
Project supported by the National Natural Science Foundation of China (No. 21572060).
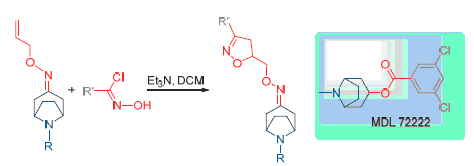
基于具有潜在杀线虫活性的托烷衍生物结构,通过1,3-偶极环加成反应,设计合成了19个未见报道的8-氮杂双环[3.2.1]辛烷-3-异噁唑肟类衍生物,通过1H NMR,13C NMR和高分辨质谱对目标化合物的结构进行表征.初步的生物活性测试表明:在浓度25mg/L下,目标化合物对南方根结线虫表现出良好的抑制活性,活性最好的化合物8-甲基-8-氮杂双环[3.2.1]辛烷-3-酮-O-((3-(4-硝基苯基)-4,5-二氢异噁唑-5-基)甲基)肟(9e)在1mg/L浓度下对根结线虫的抑制率达55.6%.
关键词: 氮杂双环; 杀线剂; 5-羟色胺; 1,3-偶极环加成反应
陆青 , 沈巧英 , 孙璐 , 王佳毅 , 宋恭华 . 8-氮杂双环[3.2.1]辛烷-3-异噁唑肟类衍生物的合成及其杀线虫活性[J]. 有机化学, 2016 , 36(4) : 760 -767 . DOI: 10.6023/cjoc201601036
Based on the potential nematicidal activities of tropane derivatives structure, 19 unreported 8-azabicyclo[3.2.1]- octane-3-isoxazole oxime derivatives were designed and synthesized by 1,3-dipolar cycloaddition reaction. Their structures were confirmed by 1H NMR, 13C NMR and HRMS techniques. Preliminary bioassay results showed that targeted compounds exhibited good nematicidal activities against Meloidogyne incognita at concentration of 25 mg/L, and 8-methyl-8-azabicyclo- [3.2.1]octan-3-one-O-((3-(4-nitrophenyl)-4,5-dihydroisoxazol-5-yl)methyl)oxime (9e) showed the best nematicidal activities with 55.6% at concentration of 1 mg/L.

Key words: 8-azabicyclo; nematicide; serotonin; 1,3-dipolar cycloaddition reaction
[1] Atkinson, H. J.; Lilley, C. J.; Urwin, P. E. Curr. Opin. Biotechnol. 2012, 23, 251.
[2] Arnot, L. F.; Veale, D. J. H.; Steyl, J. C. A.; Myburgh, J. G. J. S. Afr. Vet. Assoc. 2011, 82, 232.
[3] Souza, A. D.; Narvencar, K. P. S.; Sindhoora, K. V. J. Neurol. Sci. 2013, 335, 36.
[4] Qin, S. J.; Gan, J. Y.; Liu, W. P.; Becker, J. O. J. Agric. Food Chem. 2004, 52, 6239.
[5] Sánchez-Moreno, S.; Alonso-Prados, E.; Alonso-Prados, J. L.; García-Baudín, J. M. Pest Manage. Sci. 2009, 65, 82.
[6] Pitterna, T.; Cassayre, J.; Hüter, O. F.; Jung, P. M. J.; Maienfisch, P.; Kessabi, F. M.; Quaranta, L.; Tobler, H. Bioorg. Med. Chem. 2009, 17, 4085.
[7] Kearn, J.; Ludlow, E.; Dillon, J.; O'Connor, V.; Holden-Dye, L. Pestic. Biochem. Phys. 2014, 109, 44.
[8] Mushtaq, M.; Feely, W. F.; Syintsakos, L. R.; Wislocki, P. G, J. Agric. Food Chem. 1996, 44, 940
[9] Liu, Q. S.; Ma, X. G.; Shen, B. Y. New Cen. Agrochem. 2014, 4, 54 (in Chinese). (刘钦胜, 马新刚, 申宝玉, 今日农化, 2014, 4, 54.)
[10] Tierney, A. J. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 2001, 128, 791.
[11] Nassel, D. R. Prog. Neurobiol. 1996, 48, 325.
[12] Nassel, D. R. Prog. Neurobiol. 1988, 30, 1.
[13] Osborne, R. H. Pharmacol. Ther. 1996, 69, 117.
[14] Lange, A. B. Arch. Insect Biochem. Physiol. 2004, 56, 179.
[15] Cai, M. Y.; Li, Z.; Fan, F.; Huang, Q. C.; Shao, X. S.; Song, G. H. J. Agric. Food Chem. 2009, 58, 2624.
[16] Shen, Y.; Wang, J. Y.; Song, G. H. Mol. Diversity 2014, 18, 195.
[17] Trowell, S. C.; Saubern, S.; Liao, C. Y. US 20050054680, 2005 [Chem. Abstr. 2003, 138, 200330].
[18] Trowell, S. C.; Dumancic, M.; Liao, C. Y. Easr, P. US 20030186370, 2003 [Chem. Abstr. 2001, 135, 191974].
[19] Thompson, A. J.; Lummis, S. C. Curr. Pharm. Des. 2006, 12, 3615.
[20] Hibert, M. F.; Hoffmann, R.; Miller, R. C.; Carr, A. A. J. Med. Chem. 1990, 33, 1594.
[21] Swain, C. J.; Baker, R.; Kneen, C.; Moseley, J.; Saunders, J.; Seward, E. M.; Stevenson, G.; Beer, M.; Stanton, J.; Watling, K. J. Med. Chem. 1991, 34, 140.
[22] Song, B. A.; Liu, X. H.; Yang, S.; Hu, D. Y.; Jin, L. H.; Zhang, Y. T. Chin. J. Org. Chem. 2005, 25, 507 (in Chinese). (宋宝安, 刘新华, 杨松, 胡德禹, 金林红, 张玉涛, 有机化学 2005, 25, 507.)
[23] He, H. W.; Li, M. Q.; Huang, G. L. Pesticides 2000, 8, 4 (in Chinese). (贺红武, 李美强, 黄刚良, 农药, 2000, 8, 4.)
[24] Mihara, J.; Murata, T.; Yamazaki, D.; Yoneta, Y.; Shibuya, K.; Shimojo, E.; Görgens, U. WO 2008019760, 2008 [Chem. Abstr. 2008, 148, 262594].
[25] Li, M. Q.; He, H. W. Chem. Reag. 2001, 23, 271 (in Chinese). (李美强, 贺红武, 化学试剂, 2001, 23, 271.)
[26] Lu, T. J.; Tzeng, G. M. J. Chin. Chem. Soc. 2000, 47, 189.
[27] Koyama, J.; Sugita, T.; Suzuta, Y.; Irie, H. Chem. Pharm. Bull. 1983, 31, 2601.
[28] Kleinschmidt, R. F.; Cope, A. C. J. Am. Chem. Soc. 1944, 66, 1929.
/
| 〈 |
|
〉 |