

镍催化芳基三氟甲磺酸酯对醛的加成反应及偶联反应
收稿日期: 2016-02-03
修回日期: 2016-03-09
网络出版日期: 2016-03-18
基金资助
国家自然科学基金(No.21372202)和新世纪优秀人才支持计划(No.NCET-12-1086)资助项目.
Nickel-Catalyzed Addition and Coupling Reaction of Aryl Triflates to Aldehydes
Received date: 2016-02-03
Revised date: 2016-03-09
Online published: 2016-03-18
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372202) and the Program for New Century Excellent Talents in University (No. NCET-12-1086).
张鸣頔 , 陈斌 , 葛晨 , 刘人荣 , 高建荣 , 贾义霞 . 镍催化芳基三氟甲磺酸酯对醛的加成反应及偶联反应[J]. 有机化学, 2016 , 36(7) : 1636 -1642 . DOI: 10.6023/cjoc201602007
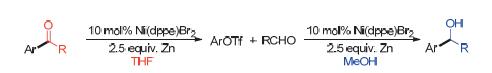
The nickel-catalyzed addition and coupling reaction between aryl triflates and aldehydes were developed. The reactions proceed smoothly in the presence of zinc powder with the use of 10 mol% Ni(dppe)Br2 as a catalyst. A range of aryl methanols and aryl ketones were isolated respectively in moderate to good yields in MeOH and THF solvent via direct addition or coupling reactions. Aliphatic and aromatic aldehydes were involved in this reaction, showing a broad substrate scope.
[1] (a) Okude, Y.; Hirano, S.; Hiyama, T.; Nozaki, H. J. Am. Chem. Soc. 1977, 99, 3179.
(b) Jin, H.; Uenishi, J.; Christ, W. J.; Kishi, Y. J. Am. Chem. Soc. 1986, 108, 5644.
(c) Takai, K.; Tagashira, M.; Kuroda, T.; Oshima, K.; Utimoto, K.; Nozaki, H. J. Am. Chem. Soc. 1986, 108, 6048.
(d) Fürstner, A.; Shi, N. J. Am. Chem. Soc. 1996, 118, 2533.
(e) Fürstner, A.; Shi, N. J. Am. Chem. Soc. 1996, 118, 12349.
[2] (a) Quan, L.-G.; Lamrani, M.; Yamamoto, Y. J. Am. Chem. Soc. 2000, 122, 4827.
(b) Pletnev, A. A.; Larock, R. C. J. Org. Chem. 2002, 67, 9428.
(c) Solé, D.; Vallverdú, L.; Solans, X.; Font-Bardía, M.; Bonjoch, J. J. Am. Chem. Soc. 2003, 125, 1587.
(d) Cacchi, S.; Fabrizi, G.; Gavazza, F.; Goggiamani, A. Org. Lett. 2003, 289.
(e) Solé, D.; Serrano, O. Angew. Chem., Int. Ed. 2007, 46, 7270.
(f) Solé, D.; Serrano, O. J. Org. Chem. 2008, 73, 9372.
(g) Zhao, Y.-B.; Mariampillai, B.; Candito, D. A.; Laleu, B.; Li, M.; Lautens, M. Angew. Chem., Int. Ed. 2009, 48, 1849.
[3] (a) Majumdar, K. K.; Cheng, C.-H. Org. Lett. 2000, 2295.
(b) Rayabarapu, D. K.; Chang, H.-T.; Cheng, C.-H. Chem. Eur. J. 2004, 10, 2991.
(c) Hsieh, J. C.; Cheng, C.-H. Chem. Commun. 2005, 4554.
(d) Hu, J.-X.; Wu, H.; Li, C.-Y.; Sheng, W.-J.; Jia, Y.-X.; Gao, J.-R. Chem. Eur. J. 2011, 17, 5234.
(e) Yin, H.; Zhao, C.; You, H.; Lin, K.; Gong, H. Chem. Commun. 2012, 7034.
(f) Wu, F.; Lu, W.; Qian, Q.; Ren, Q.; Gong, H. Org. Lett. 2012, 14, 3044.
(g) He, J.-Q.; Chen, C.; Yu, W.-B.; Liu, R.-R.; Xu, M.; Li, Y.-J.; Gao, J.-R.; Jia, Y.-X. Tetrahedron Lett. 2014, 55, 2805.
(h) Zhao, C.; Jia, X.; Wang, X.; Gong, H. J. Am. Chem. Soc. 2014, 136, 17645.
[4] Correa, A.; Martin, R. J. Am. Chem. Soc. 2014, 136, 7253.
[5] Huang, Y.-C.; Majumdar, K. K.; Cheng, C.-H. J. Org. Chem. 2002, 67, 1682.
[6] (a) Kuriyama, M.; Shimazawa, R.; Enomoto, T.; Shirai, R. J. Org. Chem. 2008, 73, 6939.
(b) Infante, R.; Nieto, J.; Andrés, C. Org. Biomol. Chem. 2011, 9, 6691.
(c) Kuriyama, M.; Ishiyama, N.; Shimazawa, R.; Onomura, O. Tetrahedron 2010, 66, 6814.
(d) Yamamoto, T.; Ohta, T.; Ito, Y. Org. Lett. 2005, 7, 4153.
(e) DeBerardinis, A. M.; Turlington, M.; Pu, L. Org. Lett. 2008, 10, 2709.
(f) Yamamoto, T.; Furusawa, T.; Zhumagazin, A.; Yamakawa, T.; Oe, Y.; Ohta, T. Tetrahedron 2015, 71, 19.
(g) Hirose, T.; Sugawara, K.; Kodama, K. J. Org. Chem. 2011, 76, 5413.
(h) Majumdar, K. K.; Cheng, C.-H. Org. Lett. 2000, 2, 2295.
[7] (a) Rao, M. L. N.; Venkatesh, V. Banerjee, D. Tetrahedron 2007, 63, 12917.
(b) Silbestri, G. F.; Masson, R. B.; Lockhart, M. T.; Chopa, A. B. J. Organomet. Chem. 2006, 619, 1520.
(c) Andrus, M. B.; Ma, Y.; Zang, Y.; Songa, C. Tetrahedron Lett. 2002, 43, 9137.
(d) Ushijima, S.; Dohi, S.; Moriyama, K.; Togo, H. Tetrahedron 2012, 68, 1436.
(e) Baghos, V. B.; Doss, S. H.; Eskander, E. F. Org. Prep. Proced. Int. 1993, 25, 301.
(f) Meng, G.; Szostak, M. Org. Lett. 2015, 17, 4364.
(g) Huang, Y. C.; Majumdar, K. K.; Cheng, C.-H. J. Org. Chem. 2002, 67, 1682.
/
| 〈 |
|
〉 |