

铁粉促进“一锅法”合成1-苯基苯并咪唑类化合物
收稿日期: 2015-12-03
修回日期: 2016-03-10
网络出版日期: 2016-03-25
基金资助
国家自然科学基金(No.21102184)和高等学校博士学科点专项科研基金(No.20110162120033)资助项目.
“One-Pot” Synthesis of 1-Phenyl-1H-benzimidazole Derivatives Facilitated by Fe
Received date: 2015-12-03
Revised date: 2016-03-10
Online published: 2016-03-25
Supported by
Project supported by the the National Natural Science Foundation of China (No. 21102184) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20110162120033).
余祖滔 , 王泽瑜 , 吴肖 , 胡高云 , 李乾斌 . 铁粉促进“一锅法”合成1-苯基苯并咪唑类化合物[J]. 有机化学, 2016 , 36(7) : 1672 -1676 . DOI: 10.6023/cjoc201512007
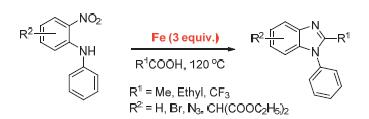
An easy and efficient “one-pot” synthetic method facilitated by Fe to synthesize substituted 1-phenyl-1H-benzimi- dazole has been developed successfully. By using liquid organic acid as solvent, 2-nitro-N-phenylaniline is converted to substituted 1-phenyl-1H-benzimidazole directly after reduction and cyclization catalyzed by Fe, with the yield of 80%~92%. Through the application of high performance liquid chromatography (HPLC) detection, the effects of the amount of solvent, the type of liquid organic acid, temperature and reaction time on the “one-pot” reaction have been fully investigated, as well as the reaction mechanism. The “one-pot” method proposed here has multiple advantages, such as mild condition, easy procedure, cheap ingredients and high yield, which provide a newly convenient synthetic route.
[1] Kaur, G.; Kaur, M.; Silakari, O. Mini-Rev. Med. Chem. 2014, 14, 747.
[2] Zhu, G. M.; Yang, L. Y.; Cui, D. M. Chin. J. Org. Chem. 2014, 34, 495 (in Chinese). (朱观明, 杨柳阳, 崔冬梅, 有机化学, 2014, 34, 495.)
[3] Whitehurst, C. B.; Sanders, M. K.; Law, M.; Wang, F. Z.; Xiong, J.; Dittmer, D. P.; Pagano, J. S. J. Virol 2013, 87, 5311.
[4] Hetzel, D. J.; Dent, J.; Reed, W. D.; Narielvala, F. M.; Mackinnon, M.; McCarthy, J. H.; Mitchell, B.; Beveridge, B. R.; Laurence, B. H.; Gibson, G. G.; Granta, A. K.; Shearmana, D.; Whiteheada, R.; Bucklea, P. Gastroenterology 1988, 95, 903.
[5] Satoh, H. Curr. Pharm. Design 2013, 19, 67.
[6] Horton, J. Fundam.-Clin. Pharmacol. 2003, 17, 205.
[7] Pawluk, S. A.; Roels, C. A.; Wilby, K. J.; Ensom, M. H. Clin. Pharmacokinet. 2015, 54, 371.
[8] Pech, P.; Heneberg, P. J. Invertebr. Pathol. 2015, 130, 61.
[9] Cheng, Z.; Zhang, Q. F.; Xu, X. L.; Li, X. N. Chin. J. Org. Chem. 2015, 35, 1189 (in Chinese). (程正, 张群峰, 许孝良, 李小年, 有机化学, 2015, 35, 1189.)
[10] Mukhopadhyay, C.; Tapaswi, P. K. Synth. Commun. 2012, 42, 2217.
[11] Muangpaisal, R.; Hung, W. I.; Lin, J. T.; Ting, S. Y.; Chen, L. Y. Tetrahedron 2014, 70, 2992.
[12] Ansari, K. F.; Lal, C. Eur. J. Med. Chem. 2009, 44, 4028.
[13] Sharghi, H.; Asemani, O.; Tabaei, S. M. H. J. Heterocycl. Chem. 2008, 45, 1293.
[14] Chari, M. A.; Shobha, D.; Kenawy, E. R.; Al-Deyab, S. S.; Reddy, B. V. S.; Vinu, A. Tetrahedron Lett. 2010, 51, 5195.
[15] Weires, N. A.; Boster, J.; Magolan, J. Eur. J. Org. Chem. 2012, 6508.
[16] Sekar, R.; Srinivasan, M.; Marcelis, A. T. M.; Sambandam, A. Tetrahedron Lett. 2011, 52, 3347.
[17] Zou, B. L.; Yuan, Q. L.; Ma, D. W. Angew. Chem., Int. Ed. 2007, 46, 2598.
[18] Selvam, K.; Swaminathan, M. Tetrahedron Lett. 2011, 52, 3386.
/
| 〈 |
|
〉 |