

稀有植物四川金粟兰化学成分的进一步研究
收稿日期: 2016-02-01
修回日期: 2016-03-04
网络出版日期: 2016-03-28
基金资助
国家自然科学基金(Nos.21472021,81273401,21575029)和国家重点基础研究发展计划(973计划,No.2013CB530700)资助项目.
Further Chemical Constituents from the Rare Chloranthaceae Plant Chloranthus sessilifolius
Received date: 2016-02-01
Revised date: 2016-03-04
Online published: 2016-03-28
Supported by
Project supported by National Natural Science Foundation of China (Nos. 21472021, 81273401, 21575029) and the National Basic Research Program of China (973 Program, No. 2013CB530700).
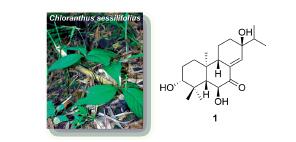
对稀有植物四川金粟兰(Chloranthus sessilifolius)全草甲醇提取物开展进一步化学成分研究,从中分离得到2个二萜类、1个苯丙素类、1个黄酮类和1个木脂素类化合物. 利用多种波谱和光谱技术(如红外、紫外、1D/2D NMR、ECD、HR-ESIMS)结合相关文献中的理化数据确定了它们的结构分别为:(3R,5S,6S,9S,10S,13S)-3,6,13-三羟基-对映松香烷-8(14)-烯-7-酮(1)、3α,15-二羟基-13α-甲氧基-对映松香烷-8(14)-烯-7-酮(2)、p-香豆酰-β-苯乙胺(3)、柚皮素三甲基醚(4)和(3R,4R,3'R,4'R)-6,6'-二甲氧基-3,4,3',4'-四氢-2H,2H'-[3,3']-双色原烯-4,4'-二醇(5). 其中,化合物1为新的对映松香烷型二萜,而3~5则均为首次从金粟兰属植物中分离得到的化合物.
王立军 , 刘彩云 , 熊娟 , 胡金锋 . 稀有植物四川金粟兰化学成分的进一步研究[J]. 有机化学, 2016 , 36(7) : 1677 -1680 . DOI: 10.6023/cjoc201602002
Two diterpenoids and a few miscellaneous components (one phenethylpropanoid, one flavonoid, and one lignan) were isolated from the MeOH extract of the whole plant of the rare Chloranthaceae plant Chloranthus sessilifolius during a further phytochemical reinvestigation. Based on their spectroscopic data (i.e., IR, UV, 1D/2D NMR, ECD, and HR-ESIMS) and/or comparing with those reported physicochemical data in related literature, the structures were determined to be (3R,5S,6S,9S,10S,13S)-3,6,13-trihydroxy-ent-abieta-8(14)-en-7-one (1), 3α,15-dihydroxy-13α-methoxy-ent-abieta-8(14)-en- 7-one (2), p-coumaroyl-β-phenethyl amine (3), narigenin trimethyl ether (4), and (3R,4R,3'R,4'R)-6,6'-dimethoxy-3,4,3',4'- tetrahydro-2H,2H'-[3,3']-bichromenyl-4,4'-diol (5). Among them, compound 1 is new, while compounds 3~5 were isolated from the Chloranthus genus for the first time.

[1] Editorial committee Flora of China, Science Press, Beijing, 1982, Vol. 20, pp. 80~96 (in Chinese). (编委会, 中国植物志, 科学出版社, 北京, 1982, Vol. 20, pp. 80~96.)
[2] Wang, A. R.; Song, H. C.; An, H. M.; Huang, Q.; Luo, X.; Dong, J. Y. Chem. Biodiversity 2015, 2, 451.
[3] Wang, L. J.; Xiong, J.; Liu, S. T.; Liu, X. H.; Hu, J.-F. Chem. Biodiversity 2014, 11, 919.
[4] Liu, S. T.; Xiong, J.; Tang, Y.; Wang, W. X.; Bui, V. B.; Ma, G. L.; Huang, Y.; Zhao, Y.; Yang, G. X.; Hu, J.-F. Chem. Biodiversity 2014, 11, 904.
[5] Xiong, J.; Liu, S. T.; Tang, Y.; Wang, W. X.; Bui, V. B.; Zhao, Y.; Fan, H.; Yang, G. X.; Hu, J.-F. Phytochem. Lett. 2013, 6, 586.
[6] Wang, D. Q. Lishizhen Med. Mater. Med. Res. 1991, 2, 140 (in Chinese). (王德群, 时珍国药研究, 1991, 2, 140.)
[7] Wang, L. J.; Xiong, J.; Liu, S. T.; Pan, L. L.; Yang, G. X.; Hu, J.-F. J. Nat. Prod. 2015, 78, 1635.
[8] Wang, L. J.; Xiong, J.; Lau, C.; Pan, L. L.; Hu, J.-F. J. Asian Nat. Prod. Res. 2015, 17, 1220.
[9] Xie, C. F.; Sun, L. M.; Liao, K. Liu, S.; Wang, M. C.; Xu, J.; Bartlam, M.; Guo, Y. Q. J. Nat. Prod. 2015, 78, 2800.
[10] Sinz, A.; Matusch, R.; Van Eläcker, F.; Santisuk, T.; Chaichana, S.; Reutrakul, V. Phytochemistry 1999, 50, 1069.
[11] Seidel, V.; Bailleul, F.; Waterman, P. G. Phytochemistry 2000, 55, 439.
[12] Sielinou, V. T.; Vardamides, J. C.; Nkengfack, A. E.; Laatsch, H. Phytochem. Lett. 2012, 5, 409.
/
| 〈 |
|
〉 |