

Cu(II)/O2体系中α-唑取代酮与2,2,6,6-四甲基哌啶-1-氧化物的α-羟氨基化反应研究
收稿日期: 2016-01-15
修回日期: 2016-03-16
网络出版日期: 2016-04-05
基金资助
国家自然科学基金(No.21272037)、广东省自然科学基金(No.S2013040014944)和广东石油化工学院大学生创新创业(No.2015DCA037)资助项目.
Researches on the α-Aminoxylation between α-Azoleketones and 2,2,6,6-Tetramethylpiperidine-1-oxyl with Cu/O2
Received date: 2016-01-15
Revised date: 2016-03-16
Online published: 2016-04-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272037), the Natural Science Foundation of Guangdong Province (No. S2013040014944) and the Guangdong University of Petrochemical Technology (No. 2015DCA037).
研究了α-唑取代酮与2,2,6,6-四甲基哌啶-1-氧化物(TEMPO)的α-羟氨基化反应. 室温条件下,以廉价二价铜盐为催化剂,以空气为氧化剂,α-唑取代酮与TEMPO发生了α-羟氨基化反应,生成了一系列烷氧基胺化合物. 该方法具有条件温和、催化剂廉价、氧化剂环保、反应效率高等特点.
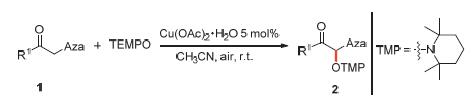
关键词: 铜催化; 氧气; α-唑取代酮; 2,2,6,6-四甲基哌啶-1-氧化物; α-羟氨基化反应
周鹏 , 邱会华 , 潘泓成 , 施继成 , 周建敏 . Cu(II)/O2体系中α-唑取代酮与2,2,6,6-四甲基哌啶-1-氧化物的α-羟氨基化反应研究[J]. 有机化学, 2016 , 36(7) : 1596 -1601 . DOI: 10.6023/cjoc201601019
The α-aminoxylation between α-azoleketones and 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) was studied in this paper. Taking Cu(II) salts as catalyst and air as oxidant, a number of alkoxyamines have been synthesized via α-aminoxylation between α-azoleketones and TEMPO in good yields at room temperature. This strategy is highlighted by appealing features such as mild reaction condition, inexpensive catalyst, green oxidant and good yield.

[1] (a) Erdmann, A.; Menon, Y.; Gros, C.; Molinier, N.; Novosad, N.; Samson, A.; Gregoire, J.; Long, C.; Ausseil, F.; Hlby, L.; Arimondo, P. B. Bioorg. Med. Chem. 2015, 23, 5946
(b) Lanier, M.; Sergienko, E.; Simão A. M.; Su, Y.; Chung, T.; Millán, J. L.; Cashman, J. R. Bioorg. Med. Chem. 2010, 18, 573
(c) Salerno, L; Modica, M. N.; Romeo, G.; Pittalà, V.; Siracusa, M. A.; Amato, M. E.; Acquaviva, R.; Giacomo, C. D.; Sorrenti, V. Eur. J. Med. Chem. 2012, 49, 118
(d) Peifer, C.; Bühler, S.; Hauser, D.; Kinkel, K.; Totzke, F.; Schächtele, C.; Laufer, S. Eur. J. Med. Chem. 2009, 44, 1788
(e) Wei, Q.-L.; Zhang, S.-S.; Gao, J.; Li, W.-H.; Xu, L.-Z.; Yu, Z.-G. Bioorg. Med. Chem. 2006, 14, 7146
(f) Pautus, S.; Sehr, P.; Lewis, J.; Fortuné, A.; Wolkerstorfer, A.; Szolar, O.; Guilligay, D.; Lunardi, T.; Décout, J.-L.; Cusack, S. J. Med. Chem. 2013, 56, 8915.
[2] (a) Hawker, C. J.; Bosmann, A. W.; Harth, E. Chem. Rev. 2001, 101, 3661.
(b) Benoit, D.; Chaplinski, V.; Braslau, R.; Hawker, C. J. J. Am. Chem. Soc. 1999, 121, 3904.
[3] (a) Sciannamea V.; Jérôme, R.; Detrembleur C. Chem. Rev. 2008, 108, 1104.
(b) Calabrese, D. R.; Ditter, D.; Liedel, C.; Blumfield, A.; Zentel, R.; Ober, C. K. ACS Macro. Lett. 2015, 4, 606.
[4] Wang, Z.-L.; An, X.-L.; Ge, L.-S.; Jin, J.-H.; Luo, X.; Deng, W.-P. Tetrahedron 2014, 70, 3788.
[5] (a) Dinca, E.; Hartmann, P.; Smr?ek, J.; Dix, I.; Jones, P. G.; Jahn, U. Eur. J. Org. Chem. 2012, 4461.
(b) Kirchberg, S.; Fröhlich, R.; Studer, A. Angew. Chem., Int. Ed. 2010, 49, 6877.
(c) Abeykoon, G. A.; Chatterjee, S.; Chen, J. S. Org. Lett. 2014, 16, 3248.
[6] Akagawa, K.; Fujiwara, T.; Sakamoto, S.; Kudo, K. Chem. Commun. 2010, 46, 8040.
[7] Bui, N.-N.; Ho, X.-H.; Mho, S.-I.; Jang, H.-Y. Eur. J. Org. Chem. 2009, 5309.
[8] Feng, P.; Song, S.; Zhang, L.-H.; Jiao, N. Synlett 2014, 25, 2717.
[9] Koike, T.; Yasu, Y.; Akita, M. Chem. Lett. 2012, 41, 999.
[10] Luo, X.; Wang, Z.-L.; Jin, J.-H.; An, X.-L.; Shen, Z.; Deng, W.-P. Tetrahedron 2014, 70, 8226.
[11] Li, Y.; Pouliot, M.; Vogler, T.; Renaud, P.; Studer, A. Org. Lett. 2012, 14, 4474.
[12] Xie, Y.-X.; Song, R.-J.; Liu, Y.; Liu, Y.-Y.; Xiang, J.-N.; Li, J.-H. Adv. Synth. Catal. 2013, 355, 3387.
[13] Selected reviews on reaction via Cu-catalyzed single electron transfer: (a) McCann, S. D.; Stahl, S. S. Acc. Chem. Res. 2015, 48, 1756.
(b) Yu, H.; Su, S.; Chi, Z.; Dang, Z. -M. Chin. J. Org. Chem. 2013, 33, 1628 (in Chinese). (于海珠, 苏圣钦, 张弛, 党智敏, 有机化学, 2013, 33, 1628.)
[14] Selected reviews on Cu-catalyzed oxidation with molecular oxygen: (a) Punniyamurthy, T.; Velusamy, S.; Iqbal J. Chem. Rev. 2005, 105, 2329.
(b) Campbell, A. N.; Stahl, S. S. Acc. Chem. Res. 2012, 45, 851.
(c) Allen, S. E.; Walvoord, R. R.; Padilla-Salinas, R.; Kozlowski, M. C. Chem. Rev. 2013, 113, 6234
Some examples on Cu-catalyzed oxidation with molecular oxygen: (d) Gao, H.; Wang, H.; Huang, Z.; Yao, L.; Peng, J.; Chen, C. Chin. J. Org. Chem. 2015, 35, 1707 (in Chinese). (高翯, 王瀚旸, 黄章杰, 姚丽萍, 彭进松, 陈春霞, 有机化学, 2015, 35, 1707.)
(e) Li, J.; Zhang, Z.; Li, C.; Luo, W.; Yang, S. Chin. J. Org. Chem. 2015, 35, 2199 (in Chinese). (李建晓, 张振明, 李春生, 罗维, 杨少容, 有机化学, 2015, 35, 2199.)
[15] (a) Tsai, A. S.; Wilson, R. M.; Harada, H.; Berqman, R. G.; Ellman, J. A. Chem. Commun. 2009, 3910
(b) Zhang, Y.; Zhang, Y.; Xiao, J.; Peng, Z.; Dong, W.; An, D. Eur. J. Org. Chem. 2015, 35, 7806.
[16] Kumar, D.; Reddy, V. B.; Kumar, A.; Mandal, D.; Tiwari, R.; Parang, K. Bioorg. Med. Chem. Lett. 2011, 21, 449.
/
| 〈 |
|
〉 |