

聚氯乙烯基紫外光吸收剂的制备及其抗光老化性能研究
Preparation of Poly(vinyl chloride)-Based UV Absorbents and Their Resistence Property of Photoaging
Received date: 2015-12-31
Revised date: 2016-03-22
Online published: 2016-04-05
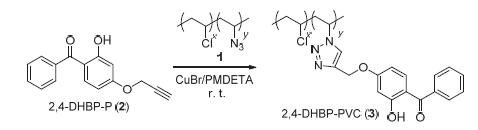
利用高效的点击反应,将小分子紫外光吸收剂接到聚氯乙烯(PVC)上,制得PVC基光稳定剂,可解决以往小分子光稳定剂与聚合物相容性差而导致的抗光老化性能持久性差和环境污染等问题. 首先,通过叠氮基的亲核取代反应制得不同取代率的叠氮化PVC(PVC-N3),2,4-二羟基二苯甲酮的4位羟基炔丙基化得到含炔基的2,4-二羟基二苯甲酮(2,4-DHBP-P). 接着,PVC-N3与2,4-DHBP-P通过铜催化的叠氮-炔基Husigen环加成反应制得紫外光吸收剂含量不同的PVC基紫外光吸收剂(2,4-DHBP-PVC). 在2,4-DHBP-PVC的紫外光辐照试验中,2,4-DHBP-PVC表现出很好的抗光老化性能,其羰基亚甲基比最低至0.03998,远远小于PVC的羰基亚甲基比(0.3331).
武国晶, 朱超, 翁枭迪, 孙晓东, 吕绪良 . 聚氯乙烯基紫外光吸收剂的制备及其抗光老化性能研究[J]. 有机化学, 2016 , 36(8) : 1963 -1969 . DOI: 10.6023/cjoc201512046
Poly(vinyl chloride) (PVC)-based light stabilizer was prepared by introducing the lower molecular UV absorbents into PVC using quantitative click reaction, which could avoid some defects (low permanence of light aging resistance property and environmental pollution) caused by poor polymer compatability of low molecular UV absorbent. Azide groups were firstly introduced into the backbone of PVC via a nucleophilic reaction to obtain PVC-N3 with different substitutive rate, and 2,4-dihydroxy-benzophenone (2,4-DHBP) was treated with propargyl bromide to prepare alkynyl-containing 2-hydroxy-4- (prop-2-ynyloxy)benzophenone (2,4-DHBP-P). Then, copper-catalyzed Husigen-Click cycloaddition reaction was performed between the pendant azide groups of PVC-N3 and alkynyl of 2,4-DHBP-P to afford the desired PVC-based UV absorbents (2,4-DHBP-PVC) with different amounts of benzophenone moieties. These 2,4-DHBP-PVC showed great resistance to photoaging while exposed to UV irradiation. Their ratio of carbonyl to methylene group were far lower than that of pure PVC (0.3331) after UV irradiating for 200 h, and the minimum value is about 0.03998.

Key words: poly(vinyl chloride); UV absorbents; photoaging
[1] Gesenhues, U. Polym. Degrad. Stab. 2000, 68, 185.
[2] Carlsson, J.; Krzymien, M.; Worsfold, J. J. Vinyl Addit. Technol. 1997, 3, 100.
[3] Naima, B. J. Vinyl Addit. Technol. 2002, 8, 45.
[4] Rabek, J. F. Polymer Photodegradation: Mechanisms and Experimental Methods, Chapman & Hall, London, UK, 1995. p.151
[5] Braun, D.; Richter, E.; Rabies, T. Angew. Makromol. Chem. 1999,271, 93.
[6] Magdy, W.; Emad, A. H.; Abir, S. Eur. Polym. J. 2005, 41, 2530.
[7] Pospisil, J.; Nespurek, S. Prog. Polym. Sci. 2000, 25, 1261.
[8] Allen, S. Polym. Photochem. 1983, 3, 167.
[9] Ormson, M.; Brown, G. Prog. React. Kinet. 1994, 19, 45.
[10] Gourrierec, D.; Ormson, M.; Brown, G. Prog. React. Kinet. 1994,19, 211.
[11] Kosower, M.; Huppert, D. Ann. Rev. Phys. Chem. 1986, 37, 127.
[12] Catalan, J.; Fabero, F.; Guijarro, S. J. Am. Chem. Soc. 1990, 112,747.
[13] Catalan, J.; Valle, C. J. Am. Chem. Soc. 1993, 115, 4321.
[14] Rieker, J.; Lemmert-Schmitt, E.; Goeller, G. J. Phys. Chem. 1992,96, 10225.
[15] Flom, R.; Barbara, F. Chem. Phys. Lett. 1983, 94, 488.
[16] Woessner, G.; Goeller, G.; Rieker, J. J. Phys. Chem. 1985, 89, 3629.
[17] Luston, J. Developments in Polymer Stabilisation-2, Applied Science Publishers, London, 1980, p. 185.
[18] Vink, P.; Scott, G. Development in Polymer Stabilization-3, Applied Science Publishers, London, 1980, p. 117.
[19] Billingham, N. C.; Calvert, P. D. Development in Polymer Stabilization-3, Applied Science Publishers, London, 1980, p. 139.
[20] Andrady, L.; Hamid, H.; Hu, X. J. Photochem. Photobiol. B 1998,46, 96.
[21] Binder, W. H.; Sachesenhofer, R. Macromol. Rapid Commun. 2007,28, 15.
[22] Wu, C. Y. Polym. Bull. 2006, 4, 76.
/
| 〈 |
|
〉 |