

Triphos配体在羧酸及其衍生物和CO2的氢化反应中的应用研究进展
收稿日期: 2016-03-08
修回日期: 2016-04-02
网络出版日期: 2016-04-07
基金资助
国家自然科学基金(Nos. 21472215,21572254)资助项目.
Advances in Hydrogenation of Carboxylic Acid Derivatives and CO2 Using Triphos as the Coordination Ligand
Received date: 2016-03-08
Revised date: 2016-04-02
Online published: 2016-04-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21472215, 21572254).
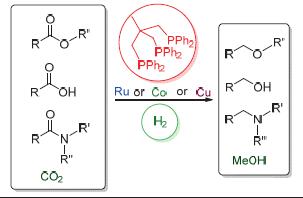
羧酸及其衍生物和二氧化碳中羰基的还原无论在基础研究中还是在工业生产上都是最重要的转化之一. 在环境问题日益严峻的今天,以氢气作为还原剂实现这些化合物的还原具有极大的吸引力. 由于均相催化反应具有反应条件温和、活性高及催化体系易于调节等优点,发展高效、高选择性的均相催化氢化体系来实现这些羰基化合物的还原成为了研究的热点. 近年来,过渡金属与不同类型配体形成的催化体系在羧酸衍生物和二氧化碳的氢化反应中的应用得到了深入的研究,取得了一些重要的进展. 其中,过渡金属与1,1,1-三(二苯基膦基甲基)乙烷(triphos)形成的催化体系在多种类型羧酸及其衍生物和二氧化碳的氢化中表现出了独特的反应活性和选择性. 本文主要介绍triphos与过渡金属钌、钴和铜形成的催化体系在羧酸及其衍生物和二氧化碳的氢化反应方面取得的进展以及相关反应机理的探讨.
张琳莉 , 韩召斌 , 张磊 , 李明星 , 丁奎岭 . Triphos配体在羧酸及其衍生物和CO2的氢化反应中的应用研究进展[J]. 有机化学, 2016 , 36(8) : 1824 -1838 . DOI: 10.6023/cjoc201603014
The reduction of carbon dioxide, carboxylic acids and their derivatives is one of the fundamental transformations both in academia and industry. Considering the increasing environmental issues, the use of molecular hydrogen as the reducing agent is especially attractive. Due to the mild reaction condition, high reactivity and easy modification of homogeneous catalysis, the development of highly efficient and selective homogeneous hydrogenation catalysts to achieve the goal is becoming a hot topic. Impressive progresses have been made using homogenous catalysts derived from transition metals and various ligands as catalysts. Among them, the catalytic system combined with a transition metal and CH3C(CH2PPh2)3 (triphos) usually shows unique reactivity and selectivity. This review will summarize the advance in the hydrogenation of carbon dioxide, carboxylic acids and their derivatives using Ru/triphos, Co/triphos and Cu/triphos as catalysts, as well as their reaction mechanisms.

[1] (a) Gunanathan, C.; Milstein, D. Chem. Rev. 2014, 114, 12024.
(b) Werkmeister, S.; Junge, K.; Beller, M. Org. Process Res. Dev. 2014, 18, 289.
(c) Pritchard, J.; Filonenko, G. A.; van Putten, R.; Hensen, E. J. M.; Pidko, E. A. Chem. Soc. Rev. 2015, 44, 3808.
[2] (a) Gribble, G. W. Chem. Soc. Rev. 1998, 27, 395.
(b) Seyden-Penne, J. Reductions by the Alumino- and Borohydrides in Organic Synthesis, 2nd ed.; Wiley, New York, 1997.
[3] (a) Noyori, R.; Ohkuma, T. Angew. Chem., Int. Ed. 2001, 40, 40.
(b) Blaser, H.-U.; Federsel, H.-J. Asymmetric Catalysis on Industrial Scale, 2nd ed., Weinheim, Wiley-VCH, 2010.
[4] McAlees, A. J.; McCrindle, R. J. Chem. Soc. C 1969, 2425.
[5] (a) Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W. A.; Kühn, F. E. Angew. Chem., Int. Ed. 2011, 50, 8510.
(b) Zhang, L.; Han, Z.; Zhao, X.; Wang, Z.; Ding, K. Angew. Chem., Int. Ed. 2015, 54, 6186.
[6] Wang, W. H.; Himeda, H.; Muckerman, J. T.; Manbeck, G. F.; Fujita, F. Chem. Rev. 2015, 115, 12936.
[7] Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, Wiley, New York, 2001.
[8] Rieke, R.; Thakur, D.; Roberts, B.; White, G. J. Am. Oil Chem. 1997, 74, 333.
[9] Stein, M.; Breit, B. Angew. Chem., Int. Ed. 2013, 52, 2231.
[10] de Vries, J. G.; Elsevier, C. J. The Handbook of Homogeneous Hydrogenation, Wiley, Weinheim, 2007.
[11] Grey, R. A.; Pez, G. P.; Wallo, A. J. Am. Chem. Soc. 1981, 103, 7536.
[12] Matteoli, U.; Menchi, G.; Bianchi, M.; Piacenti, F. J. Mol. Catal. 1988, 44, 347.
[13] Teunissen, H. T.; Elsevier, C. J. Chem. Commun. 1997, 667.
[14] Hewertson, W.; Watson, H. R. J. Chem. Soc. 1962, 1490.
[15] van Engelen, M. C.; Teunissen, H. T.; de Vries, J. G.; Elsevier, C. J. J. Mol. Catal. A: Chem. 2003, 206, 185.
[16] (a) Bianchini, C.; Meli, A.; Peruzzini, M.; Vizza, F.; Zanobini, F. Coord. Chem. Rev. 1992, 120, 193.
(b) Hierso, J.-C.; Amardeil, R.; Bentabet, E.; Broussier, R.; Gautheron, B.; Meunier, P.; Kalck, P. Coord. Chem. Rev. 2003, 236, 143.
[17] Bianchini, C.; Meli, A.; Peruzzini, M.; Vizza, F.; Frediani, P.; Ramirez, J. A. Organometallics 1990, 9, 226.
[18] Barbaro, P.; Bianchini, C.; Meli, A.; Moreno, M.; Vizza, F. Organometallics 2002, 21, 1430.
[19] (a) Bianchini, C.; Meli, A.; Moneti, S.; Vizza, F. Organometallics 1998, 17, 2636.
(b) Bianchini, C.; Masi, D.; Meli, A.; Peruzzini, M.; Vizza, F.; Zanobini, F. Organometallics 1998, 17, 2495.
(c) Bianchini, C.; Meli, A.; Vizza, F. J. Organomet. Chem. 2004, 689, 4277.
[20] Barbaro, P.; Bianchini, C.; Frediani, P.; Meli, A.; Vizza, F. Inorg. Chem. 1992, 31, 1523.
[21] Mellone, I.; Bertini, F.; Gonsalvi, L.; Guerriero, A.; Peruzzini, M. Chimia 2015, 69, 331.
[22] (a) Dub, P. A.; Ikariya, T. ACS Catal. 2012, 2, 1718.
(b) Werkmeister, S.; Neumann, J.; Junge, K.; Beller, M. Chem. Eur. J. 2015, 21, 12226.
[23] Teunissen, H. T.; Elsevier, C. J. Chem. Commun. 1998, 1367.
[24] Berke, H. Book of Abstracts, XIIth FECHEM Conference on Organometallic Chemistry, Prague, 1997, PL 9.
[25] Rosato, D. V.; Rosato, M. V. Plastic Product Material and Process Selection Handbook, Elsevier, North Holland, 2004.
[26] Furst, M. R. L.; Goff, R. L.; Quinzler, D.; Mecking, S.; Botting C. H.; Cole-Hamilon D. J. Green Chem. 2012, 12, 472.
[27] vom Stein, T.; Meuresch, M.; Limper, D.; Schmitz, M.; Hölscher; Coetzee, J.; Cole-Hamilton, D. J.; Klankermayer, J.; Leitner, W. J. Am. Chem. Soc. 2014, 136, 13217.
[28] Wesselbaum, S.; vom Stein, T.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2012, 51, 7499.
[29] Boardman, B.; Hanton, M. J.; van Rensburg, H.; Tooze, R. P. Chem. Commun. 2006, 2289.
[30] Hanton, M. J.; Tin, S.; Boardman, B. J.; Miller, P. J. Mol. Catal. A: Chem. 2011, 346, 70.
[31] Li, Y. H.; Topf, C.; Cui, X. J.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2015, 54, 5196.
[32] Kilner, M.; Tyers, D. V.; Crabtree, S. P.; Wood, M. A. US 7709689, 2003 [Chem. Abstr. 2003, 139, 366612].
[33] Crabtree, S. P.; Tyers, D. V.; Sharif, M. WO 05/051907, 2005 [Chem. Abstr. 2005, 143, 43765].
[34] Rosi, L.; Frediani, M.; Frediani, P. J. Organomet. Chem. 2010, 695, 1314.
[35] Geilen, F. M. A.; Engendahl, B.; Harwardt, A.; Marquardt, W.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2010, 49, 5510.
[36] Geilen, F. M. A.; Engendahl, B.; Hölscher, M.; Klankermayer, J.; Leitner, W. J. Am. Chem. Soc. 2011, 133, 14349.
[37] Phanopoulos, A.; White, A. J. P.; Long, N. J.; Miller, P. W. ACS Catal. 2015, 5, 2500.
[38] Cui, X. J.; Li, Y. H.; Topf, C.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2015, 54, 10596.
[39] Constable, D. J. C.; Dunn, P. J.; Hayler, J. D.; Humphrey, G. R.; Leazer, J. L.; Linderman, R. J.; Lorenz, K.; Manley, J.; Pearlman, B. A.; Wells, A.; Zaks, A.; Zhang, T. Y. Green Chem. 2007, 9, 411.
[40] Magro, A. A. N.; Eastham, G. R.; Cole-Hamilton, D. J. Chem. Commun. 2007, 3154.
[41] Dodds, D. L.; Coetzee, J.; Klankermayer, J.; Brosinski, S.; Leitner, W.; Cole-Hamilton, D. J. Chem. Commun. 2012, 48, 12249.
[42] Coetzee, J.; Dodds, D. L.; Klankermayer, J.; Brosinski, S.; Leitner, W.; Slawin, A. M. Z.; Col-Hamilton, D. J. Chem. Eur. J. 2013, 19, 11039.
[43] Cabrero-Antonino, J. R.; Alberico, E.; Junge, K.; Junge, H.; Beller M. Chem. Sci. 2016, 7, 3432.
[44] Meuresch, M.; Westhues, S.; Leitner, W.; Klankermayer, J. Angew. Chem., Int. Ed. 2016, 55, 1392.
[45] Cabrero-Antonino, J. R.; Sorribes, I.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2016, 55, 387.
[46] (a) Huff, C. A.; Sanford, M. S. J. Am. Chem. Soc. 2011, 133, 18122.
(b) Li, Y. N.; He, L. N.; Liu, A. H.; Lang, X. D.; Yang, Z. Z.; Yu, B.; Luan, C. R. Green Chem. 2013, 15, 2825.
(c) Khusnutdinova, J. R.; Garg, J. A.; Milstein, D. ACS Catal. 2015, 5, 2416.
(d) Kothandaraman, J.; Goeppert, A.; Czaun, M.; Olah, G. A.; Prakash, G. K. S. J. Am. Chem. Soc. 2016, 138, 778.
[47] Wesselbaum, S.; Moha, V.; Meuresch, M.; Brosinski, S.; Thenert, K. M.; Kothe, J.; vom Stein, T.; Englert, U.; Holscher, M.; Klankermayer, J.; Leitner, W. Chem. Sci. 2015, 6, 693.
[48] Beydoun, K.; vom Stein, T.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2013, 52, 9554.
[49] Li, Y.; Sorribes, I.; Yan, T.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2013, 52, 12156.
[50] Beydoun, K.; Ghattas, G.; Thenert, K.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2014, 53, 11010.
[51] Beydoun, K.; Thenert, K.; Streng, E. S.; Brosinski, S.; Leitner, W.; Klankermayer, J. ChemCatChem 2016, 8, 135
[52] Li, Y.; Yan, T.; Junge, K.; Beller M. Angew. Chem., Int. Ed. 2014, 53, 10476.
[53] Savourey, S.; Lefevre, G.; Berthet, J.-C.; Cantat, T. Chem. Commun. 2014, 50, 14033.
[54] Sorribes, I.; Cabrero-Antonino, J. R.; Vicent, C.; Junge, K.; Beller, M. J. Am. Chem. Soc. 2015, 137, 13580.
[55] Korstanje, T. J.; van der Vlugt, J. I.; Elsevier, C. J.; de Bruin, B. Science 2015, 350, 298.
[56] Zall, C. M.; Linehan, J. C.; Appel A. M. ACS Catal. 2015, 5, 5301.
[57] Watari, R.; Kayaki, Y.; Hirano, S.; Matsumoto, N.; Ikariya, T. Adv. Synth. Catal. 2015, 357, 1369.
/
| 〈 |
|
〉 |