

二芳基甲酮亚胺的不对称催化氢化硅烷化反应研究
收稿日期: 2015-12-30
修回日期: 2016-04-02
网络出版日期: 2016-04-07
基金资助
国家自然科学基金(No. 21172217)资助项目.
Enantioselective Hydrosilylation of N-Aryl Diaryl Ketimines
Received date: 2015-12-30
Revised date: 2016-04-02
Online published: 2016-04-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 21172217).
扈晓艳 , 胡方芝 , 张敏敏 , 廖益均 , 徐小英 , 袁伟成 , 张晓梅 . 二芳基甲酮亚胺的不对称催化氢化硅烷化反应研究[J]. 有机化学, 2016 , 36(8) : 1895 -1906 . DOI: 10.6023/cjoc201512049
Lewis base catalyzed enantioselective hydrosilylation of non-ortho-substituted N-aryl diaryl ketimines was realized. In the presence of 20 mol% of the optimal catalyst, the reactions provided a series of (diarylmethyl)amines with high yields (up to 97%) in moderate to good enantioselectivities (up to 89% ee). The absolute configuration of one product was determined by X-ray crystallographic analysis.
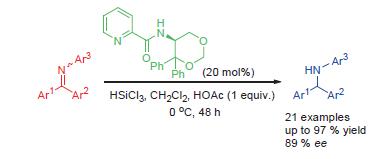
Key words: diarylmethylamines; diaryl ketimines; chiral Lewis base; hydrosilylation
[1] (a) Spencer, C. M.; Faulds, D.; Peters, D. H. Drugs 1993, 46, 1055.
(b) Bishop, M. J.; Mcnutt, R. W. Bioorg. Med. Chem. Lett. 1995, 5, 1311.
(c) Bilsky, E. J.; Calderon, S. N.; Wang, T.; Bernstein, R. N.; Davis, P.; Hruby, V. J.; Mcnutt, R. W.; Rothman, R. B.; Rice, K. C.; Porreca, F. J. Pharmacol. Exp. Ther. 1995, 273, 359.
(d) Sakurai, S.; Ogawa, N.; Suzuki, T.; Kato, K.; Ohashi, T.; Yasuda, S.; Kato, H.; Ito, Y. Chem. Pharm. Bull. 1996, 44, 765.
(e) Plobeck, N.; Delorme, D.; Wei, Z. Y.; Yang, H.; Zhou, F.; Schwarz, P.; Gawell, L.; Gagnon, H.; Pelcman, B.; Schmidt, R.; Yue, S. Y.; Walpole, C.; Brown, W.; Zhou, E.; Labarre, M.; Payza, K.; St-Onge, S.; Kamassah, A.; Morin, P. E.; Projean, D.; Ducharme, J.; Roberts, E. J. Med. Chem. 2000, 43, 3878.
(f) Gillard, M.; Van Der Perren, C.; Moguilevsky, N.; Massingham, R.; Chatelain, P. Mol. Pharmacol. 2002, 61, 391.
(g) Cetinkaya, Y.; Gocer, H.; Goksu, S.; Gulcin, I. J. Enzyme Inhib. Med. Chem. 2014, 29, 168.
[2] For a review, see: Schmidt, F.; Stemmler, R. T.; Rudolph, J.; Bolm, C. Chem. Soc. Rev. 2006, 35, 454.
[3] (a) Tomioka, K.; Inoue, I.; Shindo, M.; Koga, K. Tetrahedron Lett. 1990, 31, 6681.
(b) Delorme, D.; Berthelette, C.; Lavoie, R.; Roberts, E. Tetrahedron: Asymmetry 1998, 9, 3963.
(c) Pflum, D. A.; Krishnamurthy, D.; Han, Z. X.; Wald, S. A.; Senanayake, C. H. Tetrahedron Lett. 2002, 43, 923.
(d) Plobeck, N.; Powell, D. Tetrahedron: Asymmetry 2002, 13, 303.
(e) Han, Z. X.; Krishnamurthy, D.; Grover, P.; Fang, Q. K.; Pflum, D. A.; Senanayake, C. H. Tetrahedron Lett. 2003, 44, 4195.
(f) Cabello, N.; Kizirian, J. C.; Alexakis, A. Tetrahedron Lett. 2004, 45, 4639.
(g) Weix, D. J.; Shi, Y. L.; Ellman, J. A. J. Am. Chem. Soc. 2005, 127, 1092.
(h) Bolshan, Y.; Batey, R. A. Org. Lett. 2005, 7, 1481.
(i) Boebel, T. A.; Hartwig, J. F. Tetrahedron 2008, 64, 6824.
(j) Haurena, C.; LeGall, E.; Sengmany, S.; Martens, T. Tetrahedron 2010, 66, 9902.
(k) Han, Z. X.; Busch, R.; Fandrick, K. R.; Meyer, A.; Xu, Y. B.; Krishnamurthy, D. K.; Senanayake, C. H. Tetrahedron 2011, 67, 7035.
[4] (a) Hayashi, T.; Ishigedani, M. J. Am. Chem. Soc. 2000, 122, 976.
(b) Hermanns, N.; Dahmen, S.; Bolm, C.; Brase, S. Angew. Chem., Int. Ed. 2002, 41, 3692.
(c) Kuriyama, M.; Soeta, T.; Hao, X. Y.; Chen, O.; Tomioka, K. J. Am. Chem. Soc. 2004, 126, 8128.
(d) Tokunaga, N.; Otomaru, Y.; Okamoto, K.; Ueyama, K.; Shintani, R.; Hayashi, T. J. Am. Chem. Soc. 2004, 126, 13584.
(e) Hayashi, T.; Kawai, M.; Tokunaga, N. Angew. Chem., Int. Ed. 2004, 43, 6125. .
(f) Otomaru, Y.; Tokunaga, N.; Shintani, R.; Hayashi, T. Org. Lett. 2005, 7, 307.
(g) Cabello, N.; Kizirian, J. C.; Gille, S.; Alexakis, A.; Bernardinelli, G.; Pinchard, L.; Caille, J. C. Eur. J. Org. Chem. 2005, 4835.
(h) Jagt, R. B. C.; Toullec, P. Y.; Geerdink, D.; de Vries, J. G.; Feringa, B. L.; Minnaard, A. D. J. Angew. Chem., Int. Ed. 2006, 45, 2789.
(i) Duan, H. F.; Jia, Y. X.; Wang, L. X.; Zhou, Q. L. Org. Lett. 2006, 8, 2567-2569.
(j) Wang, Z. Q.; Feng, C. G.; Xu, M. H.; Lin, G. Q. J. Am. Chem. Soc. 2007, 129, 5336.
(k) Clayden, J.; Dufour, J.; Grainger, D. M.; Helliwell, M. J. Am. Chem. Soc. 2007, 129, 7488.
(l) Nakagawa, H.; Rech, J. C.; Sindelar, R. W.; Ellman, J. A. Org. Lett. 2007, 9, 5155.
(m) Marelli, C.; Monti, C.; Gennari, C.; Piarulli, U. Synlett 2007, 2213.
(n) Okamoto, K.; Hayashi, T.; Rawal, V. H. Chem. Commun. 2009, 4815.
(o) Hao, X. Y.; Kuriyama, M.; Chen, Q.; Yamamoto, Y.; Yamada, K.; Tomioka, K. Org. Lett. 2009, 11, 4470.
(p) Shintani, R.; Takeda, M.; Tsuji, T.; Hayashi, T. J. Am. Chem. Soc. 2010, 132, 13168.
(q) Cao, Z. P.; Du, H. F. Org. Lett. 2010, 12, 2602.
(r) Shintani, R.; Soh, Y. T.; Hayashi, T. Org. Lett. 2010, 12, 4106.
(s) Shao, C.; Yu, H. J.; Wu, N. Y.; Feng, C. G.; Lin, G. Q. Org. Lett. 2010, 12, 3820.
(t) Wang, L.; Wang, Z. Q.; Xu, M. H.; Lin, G. Q. Synthesis 2010, 3263.
(u) Crampton, R.; Woodward, S.; Fox, M. Adv. Synth. Catal. 2011, 353, 903.
(v) Hao, X. Y.; Chen, Q.; Kuriyama, M.; Yamada, K.; Yamamoto, Y.; Tomioka, K. Catal. Sci. Technol. 2011, 1, 62.
(w) Shintani, R.; Narui, R.; Tsutsumi, Y.; Hayashi, S.; Hayashi, T. Chem. Commun. 2011, 47, 6123.
(x) Han, W. Y.; Wu, Z. J.; Zhang, X. M.; Yuan, W. C. Org. Lett. 2012, 14, 976.
(y) Han, W. Y.; Zuo, J.; Zhang, X. M.; Yuan, W. C. Tetrahedron 2013, 69, 537.
[5] (a) Malkov, A. V.; Vrankova, K.; Ston?ius, S.; Ko?ovsky, P. J. Org. Chem. 2009, 74, 5839.
(b) Hou, G. H.; Tao, R.; Sun, Y.; Zhang, X. M.; Gosselin, F. J. Am. Chem. Soc. 2010, 132, 2124.
(c) Nguyen, T. B.; Wang, Q.; Gueritte, F. Chem. Eur. J. 2011, 17, 9576.
(d) Thanh, B. N.; Bousserouel, H.; Wang, Q. A.; Gueritte, F. Adv. Synth. Catal. 2011, 353, 257.
(e) Amezquita-Valencia, M.; Cabrera, A. J. Mol. Catal. A: Chem. 2013, 366, 17.
(f) Hu, X. Y.; Zhang, M. M.; Shu, C.; Zhang, Y. H.; Liao, L. H.; Yuan, W. C.; Zhang, X. M. Adv. Synth. Catal. 2014, 356, 3539.
[6] Chu, L.; Wang, X. C.; Moore, C. E.; Rheingold, A. L.; Yu, J. Q. J. Am. Chem. Soc. 2013, 135, 16344.
[7] For some recent reviews, see: (a) Zhou, Q. L.; Xie, J. H. Top. Curr. Chem. 2014, 343, 75.
(b) Li, X. H.; Wu, P. Curr. Org. Chem. 2014, 18, 1242-1261.
(c) Ji, Y. G.; Wu, L.; Fan, Q. H. Acta Chim. Sinica 2014, 72, 798 (in Chinese).
(季益刚, 吴磊, 范青华, 化学学报, 2014, 72, 798.)
(d) He, Y. M.; Song, F. T.; Fan, Q. H. Top. Curr. Chem. 2014, 343, 145.
(e) Wang, D.; Hou, C. J.; Chen, L. F.; Liu, X. N.; An, Q. D.; Hu, X. P. Chin. J. Org. Chem. 2013, 33, 1355 (in Chinese).
(王东, 侯传金, 陈丽凤, 刘小宁, 安庆大, 胡向平, 有机化学, 2013, 33, 1355.)
(f) Chen, Q. A.; Ye, Z. S.; Duan, Y.; Zhou, Y. G. Chem. Soc. Rev. 2013, 42, 497.
(g) Etayo, P.; Vidal-Ferran, A. Chem. Soc. Rev. 2013, 42, 728.
(h) Xie, J. H.; Zhu, S. F.; Zhou, Q. L. Chem. Soc. Rev. 2012, 41, 4126.
(i) Xie, J. H.; Zhou, Q. L. Acta Chim. Sinica 2012, 70, 1427 (in Chinese).
(谢建华, 周其林, 化学学报, 2012, 70, 1427.)
(j) Nugent, T. C.; El-Shazly, M. Adv. Synth. Catal. 2010, 352, 753.
(k) Cheng, T. Y.; Zhao, Q. K.; Zhang, D. C.; Liu, G. H. Curr. Org. Chem. 2015, 19, 667.
(l) Foubelo, F.; Yus, M. Chem. Rec. 2015, 15, 907.
(m) Zheng, C.; You, S. L. Chem. Soc. Rev. 2012, 41, 2498.
(n) Robertson, A.; Matsumoto, T.; Ogo, S. Dalton Trans. 2011, 40, 10304.
(o) Tang, Y. F.; Deng, J. G. Prog. Chem. 2010, 22, 1242.
(p) Wang, Z. Y.; Jiang, Z. J. Asian J. Chem. 2010, 22, 4141.
(q) Jones, S.; Warner, C. J. A. Org. Biomol. Chem. 2012, 10, 2189.
(r) Weickgenannt, A.; Oestreich, M. ChemCatChem 2011, 3, 1527.
(s) Guizzetti, S.; Benaglia, M. Eur. J. Org. Chem. 2010, 5529.
[8] Zheng, H. J.; Deng, J. G.; Lin, W. Q.; Zhang, X. M. Tetrahedron Lett. 2007, 48, 7934.
[9] Jiang, Y.; Chen, X.; Zheng, Y. S.; Xue, Z. Y.; Shu, C.; Yuan, W. C.; Zhang, X. M. Angew. Chem., Int. Ed. 2011, 50, 7304.
[10] Chen, X.; Hu, X. Y.; Shu, C.; Zhang, Y. H.; Zheng, Y. S.; Jiang, Y.; Yuan, W. C.; Liu, B.; Zhang, X. M. Org. Biomol. Chem. 2013, 11, 3089.
[11] Xue, Z. Y.; Jiang, Y.; Yuan, W. C.; Zhang, X. M. Eur. J. Org. Chem. 2010, 616.
[12] Zheng, H. J.; Deng, J. G.; Lin, W. Q.; Zhang, X. M. Tetrahedron Lett. 2007, 48, 7934.
[13] CCDC 1038813 (for 2a) contains the supplementary crystallo graphic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www. ccdc. cam. ac. uk/data_request/cif.
[14] Artico, M.; De Martino, G.; Pasquali, A. D.; Regina, G. L.; Silvestri, R.; Ragno, R.; Bergamini, A.; Ciaprini, C.; Sinistro, A.; Crespan, E.; Maga, G. J. Med. Chem. 2003, 46, 2482.
[15] Sato, M.; Nagashima, S.; Murakami, M.; Kaneko, C. Tetrahedron 1993, 49, 6575.
/
| 〈 |
|
〉 |