

无溶剂、无催化剂有效合成二苯基-2,4-氧杂-8,10-氮杂螺[5.5]十一烷-1,5,9-三酮衍生物
收稿日期: 2016-01-23
修回日期: 2016-03-22
网络出版日期: 2016-04-07
基金资助
国家自然科学基金(No. 20566004)和江西省研究生创新基金(No. YC2015-B023)资助项目.
Efficient One-Pot Synthesis of Diphenyl-2,4-dioxa-8,10-diazaspiro-[5.5]undecane-1,5,9-trione Derivatives under Solvent-Free and Catalyst-Free
Received date: 2016-01-23
Revised date: 2016-03-22
Online published: 2016-04-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20566004) and the Graduate Innovation Foundation of Jiangxi Province (No. YC2015-B023).
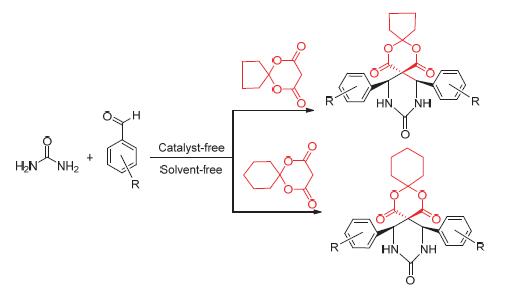
在无溶剂和无催化剂条件下,以芳香醛、2,2-亚丁基-1,3-二噁烷-4,6-二酮或2,2-亚戊基-1,3-二噁烷-4,6-二酮和尿素为原料,经三组分反应合成了12种二苯基-2,4-氧杂-8,10-氮杂螺[5.5]十一烷-1,5,9-三酮衍生物. 反应无溶剂污染,反应条件温和,收率为49%~63%. 此外,还讨论了反应速度与取代基的关系,探讨了可能的缩合反应机理,并应用1H NMR、13C NMR、IR及ESI-MS等技术手段确定了产品的结构.
许招会 , 周鹏 , 林春花 , 刘德永 . 无溶剂、无催化剂有效合成二苯基-2,4-氧杂-8,10-氮杂螺[5.5]十一烷-1,5,9-三酮衍生物[J]. 有机化学, 2016 , 36(8) : 1948 -1953 . DOI: 10.6023/cjoc201601032
Twelve kinds of diphenyl-2,4-dioxa-8,10-diazaspiro[5.5]undecane-1,5,9-trione derivatives were synthesized by the three-component one-pot reaction of aromatic aldehydes with urea and 2,2-butylidene-1,3-dioxane-4,6-dione or 2,2-pentyli- dene-1,3-dioxane-4,6-dione under solvent-free and catalyst-free. The yields ranged from 49% to 63%. Its advantages were no solvent pollution and mild reaction conditions. Furthermore, a proposed reaction mechanism for this condensation reaction was speculated and the relationship between reaction speed and substituent groups was also described. All the compounds synthesized were confirmed by 1H NMR, 13C NMR, ESI-MS and IR techniques.

[1] Arimoto, H.; Hayakawa, I.; Kuramoto, M.; Uemure, D. Tetrahedron Lett. 1998, 39, 861.
[2] Chou, T.; Kuramoto, M.; Otani, Y.; Shkano, M.; Yazawa, K.; Uemere, D. Tetrahedron Lett. 1996, 37, 3867.
[3] Rovnyak, G. C.; Kimball, S. D.; Barbara, B.; Gabriella, C.; John, D. D.; Jack, G.; Anders, H.; Mary, M.; James P. M. J. Med. Chem. 1995, 38, 119.
[4] Snider, B. B.; Shi, Z. J. Org. Chem. 1993, 58, 3828.
[5] Wessjohann, L. A.; Rivera, D. G.; Vercillo, O. E. Chem. Rev. 2009, 109, 796.
[6] Xiao, L. W.; Peng, X. X.; Zhou, Q. X.; Kou, W.; Shi, Y. R. Chin. J. Org. Chem. 2015, 35, 1204 (in Chinese).
(肖立伟, 彭晓霞, 周秋香, 寇伟, 时亚茹, 有机化学, 2015, 35, 1204.)
[7] Yang, K.; Xiang, J. B.; Bao, G. C.; Dang, Q.; Bai, X. ACS Comb. Sci. 2013, 15, 519.
[8] Dipak, P.; Debajyoti, B.; Mukut, G.; Hu, W. H. Mol. Divers. 2011, 15, 257.
[9] Srinivasa, R. J.; Divya, V.; Shubha, J. J. Chem. Pharm. Res. 2012, 4, 2373.
[10] Zhu, Y. L.; Huang, S. L.; Pan, Y. J. Eur. J. Org. Chem. 2005, 2354.
[11] Laishram, R. D.; Okram, M. S. Indian J. Chem. 2012, 51B, 1426.
[12] Anil, S.; Sanjay, K.; Jagir S. S. Indian J. Chem., 2004, 43B, 2482.
[13] Xu, Z. H.; Tu, Y. H. Chin. J. Org. Chem. 2015, 35, 1357 (in Chinese).
(许招会, 涂缘鸿, 有机化学, 2015, 35, 1357.)
[14] Naser, M.; Khalil, P.; Masoud, B.; Soudabeh, K. Asian J. Chem. 2013, 25, 3373.
[15] Wang, R.; Liu, Z. Q. J. Org. Chem. 2012, 77, 3952.
[16] Laurence, C.; Loïc, P.; Jérôme, M.; Vincent, C.; Xavier, M.; Christine, G. J. Org. Chem., 2015, 80, 595.
[17] Zeba, N. S.; Kulsum, K. ACS Sustainable Chem. Eng. 2014, 2, 1187.
[18] Wang, R.; Liu, Z. Q. J. Org. Chem. 2012, 77, 3952.
[19] Marcos, A. P. M.; Clarissa, P. F.; Dayse, N. M.; Lilian, B.; Pablo, M. Chem. Rev. 2009, 109, 4140.
[20] Maneeporn, P.; Romain, R.; Miho, H.; Waraporn, P.; Vudhichai, P.; Keiji, M. J. Org. Chem. 2015, 80, 6959.
[21] Haline, G. O. A.; Tatiani, B. L.; Aline, L. O.; Heibbe C. B. Oliveira.; Fabricio, M. S.; Fabio, C. G.; Roberto, Y. S.; Wender, A. Silva.; Brenno, A. D. N. J. Org. Chem. 2014, 79, 3383.
[22] Zheng, W. R.; Fu, Y.; Liu, L.; Guo, Q. X. Acta Phys.-Chim. Sin. 2007, 23, 1018 (in Chinese).
(郑文锐, 傅尧, 刘磊, 郭庆祥, 物理化学学报, 2007, 23, 1018.)
[23] Yan, N.; Xiong, B.; Liao, W. L.; Xu, Z. H. Chin. J. Org. Chem. 2010, 30, 1391 (in Chinese).
(严楠, 熊斌, 廖维林, 许招会, 有机化学, 2010, 30, 1391.)
/
| 〈 |
|
〉 |