

N-氯代丁二酰亚胺与异腈的双氯化反应制备N-苯基二氯亚胺类化合物
收稿日期: 2016-01-25
修回日期: 2016-03-30
网络出版日期: 2016-04-07
基金资助
国家自然科学基金(Nos. 21172197和21372201)及浙江工业大学“省重中之重一级学科”开放基金资助项目.
Dichlorinating Reaction of N-Chlorosuccinimide with Isonitriles for the Synthesis of N-Phenyldichloroimides
Received date: 2016-01-25
Revised date: 2016-03-30
Online published: 2016-04-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172197 and 21372201) and the Opening Foundation of Zhejiang Key Course of Chemical Engineering and Technology, Zhejiang University of Technology.
任少波 , 张海峰 , 张剑 , 张巍 , 刘运奎 . N-氯代丁二酰亚胺与异腈的双氯化反应制备N-苯基二氯亚胺类化合物[J]. 有机化学, 2016 , 36(8) : 1954 -1957 . DOI: 10.6023/cjoc201601033
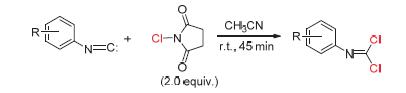
With N-chlorosuccinimide as the chlorinating source, the dichlorination of isonitriles has been achieved to afford N-phenyldichloroimide compounds under mild and convenient conditions. The optimized reaction conditions were established through systematic investigations of the effect of solvents, temperature, time, chlorinating sources and their dosages in the reaction. The reaction exhibits good compatibility of various substrates, and a variety of isonitriles with different substituted patterns can undergo the dichlorination smoothly and furnish products with high yields of up to 93%.
Key words: isonitrile; N-chlorosuccinimide; N-phenyldichloroimide; dichlorination
[1] Liu, W.; Tan, L.; Zhou, P.; Chen, C.; Zhang Q. Synthesis 2013, 45, 2600.
[2] (a) Park, J. N.; Ko, S. Y.; Koh, H. Y. Tetrahedron Lett. 2000, 41, 5553.
(b) Loncaric, C.; Wulff, W. D. Org. Lett. 2001, 3, 3675.
[3] (a) Wang, Z.-H.; Zheng, C.; Li, F.; Zhao, L.; Chen, F.-E.; He, Q.-Q. Synthesis 2012, 44, 699.
(b) Wu, G.; Schumacher, D. P.; Tormos, W.; Clark, J. E.; Murphy, B. L. J. Org. Chem. 1997, 62, 2996.
(c) Wang, Z.; Li, F.; Zhao, L.; He, Q.; Chen, F.; Zheng, C. Tetrahedron 2011, 67, 9199.
(d) Lu, W.; Chen, P.; Lin, G. Tetrahedron 2008, 64, 7822.
[4] Duan, X.; Zhou, H.; Tian, X.; Liu, J.; Ma, J. Synthesis 2015, 47, 777.
[5] Jason, T.; Richard, T.; Graham, K. M. J. Am. Chem. Soc. 2013, 135, 16312.
[6] Kaim, L.; Grimaud, L.; Patil, P. Org. Lett. 2011, 13, 1261.
[7] Nef, U. Liebigs Ann. Chem. 1892, 270, 267.
[8] Claire, M.; Gober, H.; Le, B. Tetrahedron Lett. 2012, 53, 4536.
[9] Wang, Q.; Dong, X.; Xiao, T.; Zhou, L. Org. Lett. 2013, 15, 4846.
[10] Xia, Z.; Huang, J.; He, Y.; Zhao, J.; Lei, J.; Zhu, Q. Org. Lett. 2014, 16, 2546.
[11] Wang, L.; Sha, W.; Dai, Q.; Feng, X.; Wu, W.; Peng, H.; Chen, B.; Cheng, J. Org. Lett. 2014, 16, 2088.
[12] Leifert, D.; Daniliuc, C.; Studer, A. Org. Lett. 2013, 15, 6286.
[13] Zhang, B.; Studer, A. Org. Lett. 2014, 16, 3990.
[14] Liu, J.; Fan, C.; Yin, H.; Qin, C.; Zhang, G.; Zhang, X.; Yi, H.; Lei, A. Chem. Commun. 2014, 50, 2145.
[15] Kühle, E.; Anders, B.; Zumach, G. Angew. Chem. 1967, 79, 663.
[16] Tadeusz, T.; Janusz, S.; Dariusz, M. Acta Pol. Pharm. 1988, 45, 387.
/
| 〈 |
|
〉 |