

芳香醛与五氯化磷氯化反应的研究
Study on Chlorination Reaction of Aromatic Aldehydes with Phenylphosphonic Dichloride
Received date: 2016-01-31
Revised date: 2016-03-14
Online published: 2016-04-07
吴江 , 周俊鹏 , 石亚磊 , 朱锦桃 . 芳香醛与五氯化磷氯化反应的研究[J]. 有机化学, 2016 , 36(8) : 1958 -1962 . DOI: 10.6023/cjoc201601046
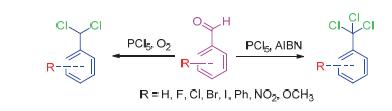
A conversion of aromatic aldehydes to dichloromethylated or trichloromethylated aromatics has been firstly accomplished by using phosphorus pentachloride as the chlorination agent. In this reaction, dichloromethylated aromatics can be obtained alone when it keeps constantly blowing oxygen into the reaction solution, and 2-methylpropionitrule can also be used to promote the formation of trichloromethylated aromatics. And it has provided a new method of highly efficient synthesis for dichloromethylated aromatics and trichloromethylated aromatics in good yields.
[1] Joshi, A. V. Synth Commun. 2005, 35, 2715.
[2] Lei, C.-H. Org. Chem. Front. 2014, 1, 909
[3] (a) Zhao, J. Zhejiang Chem. Ind. 1997, 28, 3 (in Chinese).
(赵军, 浙江化工, 1997, 28, 3.)
(b) Chen, L.-X. Mod. Agrochem. 2004, (3), 3 (in Chinese).
(程隆新, 现代农药, 2004, (3), 3.)
(c) Qin, F.-L. Chin. J. Org. Chem. 2012, 32, 7 (in Chinese).
(卿凤翎, 有机化学, 2012, 32, 7.)
(d) Bloodworth, A. J. Tetrahedron Lett. 1987, 28, 5347.
[4] Xu, D.-Q. Agrochemicals 2001, 40, 9 (in Chinese).(许丹倩, 农药, 2001, 40, 9.)
[5] (a) Fergus, S. J. Org. Chem. 2004, 69, 4663.
(b) Yakubov, A. P. Tetrahedron 1993, 49, 3397.
(c) Han, G. Y. J. Org. Chem. 1981, 46, 4695.
(d) Schiess, P. Tetrahedron Lett. 1982, 23, 3669.
[6] Koerbe, S. Inorg. Chem. 2004, 43, 8158.
[7] Tang, X.-P. Tetrahedron Lett. 2014, 55, 799.
[8] (a) Xia, X.-S. Beil. J. Org. Chem. 2014, 10, 1397.
(b) An, J. Tetrahedron 2013,69, 8769.
[9] Kabalka, G. W. Tetrahedron Lett. 2000, 41, 579.
[10] (a) Firouzabadi, H.; Shiriny, F. Tetrahedron 1996, 52, 14929.
(b) Jung, M. E.; Wasserman, J. I. Tetrahedron Lett. 2003, 44, 7273.
[11] Chen, H.-B. Chin. J. Org. Chem. 2005, 25, 532 (in Chinese).
(陈红飙, 有机化学, 2005, 25, 532.)
/
| 〈 |
|
〉 |