

相转移催化法和手性催化加氢法立体选择性地合成Fmoc保护的(S)-3,5-二溴苯丙氨酸
收稿日期: 2016-01-31
修回日期: 2015-03-17
网络出版日期: 2016-04-07
基金资助
国家自然科学基金面上(No.21272029)资助项目.
Synthesis of Fmoc-protected (S)-3,5-Dibromophenylalanine in the Presence of a Phase Transfer Catalyst or a Chiral Catalyst
Received date: 2016-01-31
Revised date: 2015-03-17
Online published: 2016-04-07
Supported by
Project supported by the National Natural Science Foundation of China (No.21272029).
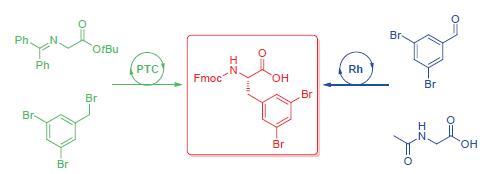
通过两种不对称催化方法合成了(S)-3,5-二溴苯丙氨酸.一种方法是以二苯亚胺甘氨酸叔丁酯和3,5-二溴苄基溴为底物,在O-烯丙基-N-9-蒽甲基溴化辛可宁定催化下,经不对称烷基化反应得到了(S)-3,5-二溴苯丙氨酸的衍生物,ee值达到94.9%,重点优化了不对称相转移催化烷基化反应的条件,得到了最优反应条件.另一种方法是以2-乙酰胺基-3-(3,5-二溴苯基)丙烯酸为底物,在双(1,5-环辛二烯)-三氟甲磺酸铑(I)和(R)-N-二苯基膦-N-甲基-(S)-2-(二苯基膦)二茂铁基乙胺催化下加氢得到乙酰基保护的(S)-3,5-二溴苯丙氨酸,再进行水解反应,最终得到(S)-3,5-二溴苯丙氨酸.经Fmoc的保护,得到Fmoc保护的(S)-3,5-二溴苯丙氨酸,ee值达到94.7%.所述两种方法中,第一种方法产率较高,对映选择性也较高,适合应用于其他手性二卤代苯丙氨酸的合成.
关键词: (S)-3,5-二溴苯丙氨酸; 相转移催化; 不对称催化氢化; 烷基化
王沁婷 , 赵帅 , 金雷 , 陈新 . 相转移催化法和手性催化加氢法立体选择性地合成Fmoc保护的(S)-3,5-二溴苯丙氨酸[J]. 有机化学, 2016 , 36(9) : 2242 -2246 . DOI: 10.6023/cjoc201601045
Two methods of catalytic asymmetric synthesis of (S)-3,5-dibromophenylalanine are presented. One approach is to use asymmetric alkylation reaction starting from diphenylimine glycine tert-butyl ester and 3,5-dibromobenzyl bromide, with O-allyl-N-9-anthracene methyl bromide cinchonidine as phase-transfer catalyst, the (S)-3,5-dibromophenylalanine derivative was obtained (up to 94.9% ee). The optimized conditions of asymmetric phase transfer catalytic alkylation are explored. Another method is to employ asymmetric hydrogenation starting from 2-acetylamino-3-(3,5-dibromophenyl)acrylic acid with bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate and (R)-diphenylphosphino-N-methyl-1-[(S)-2-diphenyl-phos-phino)ferrocenyl]ethylamine[(R)-methyl BoPhoz] as chiral catalyst. (S)-3,5-Dibromophenylalanine hydrochloride was obtained after hydrolysis. By Fmoc protection, Fmoc-(S)-3,5-dibromophenylalanine was obtained (up to 94.7% ee). By comparison of the two methods, the first one gives higher overall yield and a little bit better selectivity, and is more suitable for the synthesis of other chiral dihalo-substituented phenylalanine derivatives.

[1] Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937.
[2] Chalmers, J. R.; Dickson, G. T.; Elks, J.; Hems, B. A. J. Chem. Soc. 1949, 3424.
[3] Matsuura, T.; Cahnmann, H. J. J. Am. Chem. Soc. 1959, 81, 871.
[4] Jordan, D. G.; Eduardo, S. S.; Robert, A. B. Org. Lett. 2015, 17, 2182.
[5] Farrell, D. J.; Couturier, C.; Hryniewicz, W. Int. J. Antimicrob. Agents 2008, 31, 245.
[6] Stephen, G.; David, J. Emerging Infect. Dis. 2009, 15, 1260.
[7] Wang, J. B.; Seungkirl, A.; Alem, W. K.; Liu, R.; Ren, J.; Hu, K.; Sun, X. Q.; Chen, X. Chin. J. Org. Chem. 2013, 33, 634(in Chinese). (王江波, Seungkirl Ahn, Alem W. Kahsai, 刘蓉, 任杰, 胡昆, 孙小强, 陈新, 有机化学, 2013, 33, 634.)
[8] Parmeggiani, F.; Lovelock, S. L.; Weise, N. J.; Ahmed, S. T.; Turner, N. J. Angew. Chem., Int. Ed. 2015, 54, 4608.
[9] Gloge, A.; Zon, J.; Kovari, A.; Poppe, L.; Retey, J. Chem. Eur. J. 2000, 6, 3386.
[10] Luo, Y. C.; Zhang, H. H.; Wang, Y.; Xu, P. F. Acc. Chem. Res. 2010, 43, 1317.
[11] Takeda, R.; Kawamura, A.; Kawashima, A.; Sato, T.; Moriwaki, H.; Izawa, K.; Akaji, K.; Wang, S.; Liu, H.; Aceña, J. L.; Soloshonok, V. A. Angew. Chem., Int. Ed. 2014, 53, 12214.
[12] Corey, E. J.; Feng, X.; Noe, M. C. J. Am. Chem. Soc. 1997, 119, 12414.
[13] William, D. B.; Xu, F.; Noe, M. C. J. J. Am. Chem. Soc. 1989, 111, 2353.
[14] Shirakawa, S. J.; Maruoka, K. J. Angew. Chem., Int. Ed. 2013, 52, 4312
[15] Nun, P.; Perez, V.; Calmes, M.; Martinez, J. Chem. Eur. J. 2012, 18, 3773.
[16] Chinchilla, R.; Najera, C.; Ortega, F. J. Eur. J. Org. Chem. 2007, 6034
[17] Wu, Y. C.; Bernadat, G.; Masson, G.; Couturier, C. J. Org. Chem. 2009, 74, 2046.
[18] Liu, J.; Deng, X. H.; Fitzgerald, A. E.; Sales. Z. S. Org. Biomol. Chem. 2011, 9, 2654.
[19] O'Donnel, M. J.; Boniece, J. M.; Earp, S. E. Tetrahedron Lett. 1978, 30, 2641.
[20] Corey, E. J.; Xu, F.; Noe, M. C. J. Am. Chem. Soc. 1997, 119, 12414.
[21] McAllister, L. A.; Bechle, B. M.; Dounay, A. B. J. Org. Chem. 2011, 76, 3484.
[22] Zheng, B. H.; Ding, C. H.; Hou, X. L.; Dai, L. X. Org. Lett. 2010, 12, 1688.
/
| 〈 |
|
〉 |