

无催化剂条件下合成喹啉并吡咯并喹啉衍生物
收稿日期: 2016-02-01
修回日期: 2016-03-25
网络出版日期: 2016-04-15
基金资助
江苏省高校重大基础研究基金(No. 14KJA150004)和江苏省高校优势学科建设工程资助项目.
Synthesis of Quinopyrroloquinoline Derivatives under Catalyst-Free Conditions
Received date: 2016-02-01
Revised date: 2016-03-25
Online published: 2016-04-15
Supported by
Project supported by the Major Natural Science Foundation of Jiangsu Province (No. 14KJA150004), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
赵群 , 姚昌盛 , 王香善 . 无催化剂条件下合成喹啉并吡咯并喹啉衍生物[J]. 有机化学, 2016 , 36(8) : 1932 -1936 . DOI: 10.6023/cjoc201602001
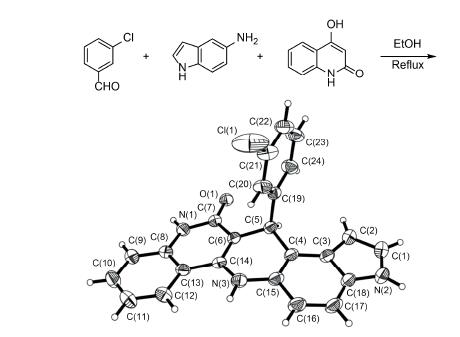
In this paper, a series of 13-aryl-6,13-dihydro-3H-quino[4,3-b]pyrrolo[3,2-f]quinolin-12(11H)-one derivatives was synthesized in EtOH under catalyst-free conditions using aromatic aldehydes, 5-aminoindole and 4-hydroxyquinolin-2(1H)- one as reactants. The structure of 4b was confirmed by X-ray diffraction analysis. This procedure has the advantages of simplicity and easy operation. An efficient method for the synthesis of fused pentacyclic heterocycles containing biquinolines moieties is provided.
Key words: quinopyrroloquinoline; 5-aminoindole; catalyst-free; synthesis
[1] (a) Keri, R. S.; Patil, S. A. Biomed. Pharmacother. 2014, 68, 1161.
(b) Muregi, F. W.; Wamakima, H. N.; Kimani, F. T. Curr. Pharm. Des. 2012, 18, 3505.
(c) Chauhan, S. S.; Sharma, M.; Chauhan, P. M. S. Drug News Perspect 2010, 23, 632.
(d) Weissbuch, I.; Leiserowitz, L. Chem. Rev. 2008, 108, 4899.
[2] Pascolo, S. Eur. J. Pharm. 2016, 771, 139.
[3] Babalonis, S.; Hampson, A. J.; Lofwall, M. R.; Nuzzo, P. A.; Walsh, S. L. J. Clin. Pharm. 2015, 55, 1332.
(a) Zhang, S.-S.; Jiang, C.-Y.; Wu, J.-Q.; Liu, X.-G.; Li, Q.; Huang, Z.-S.; Li, D.; Wang, H. Chem. Commun. 2015, 51, 10240.
(b) Tan, C.; Xiang, H.; He, Q.; Yang, C. Eur. J. Org. Chem. 2015, (17), 3656.
(c) Heravi, M. M.; Hosseini, M.; Oskooie, H. A.; Baghernejad, B.; Farzaneh, F. Chin. J. Chem. 2010, 28, 2045.
(d) Ranjbar-Karimi, R.; Hashemi-Uderji, S.; Bazmandegan-Sha- mili, A. Chin. J. Chem. 2011, 29, 1624.
(e) Du, B. X.; Li, Y. L.; Wang, X. S.; Zhang, M. M.; Shi, D. Q.; Tu, S. J. Chin. J. Org. Chem. 2006, 26, 698 (in Chinese).
(杜百祥, 李玉玲, 王香善, 张梅梅, 史达清, 屠树江, 有机化学, 2006, 26, 698.)
(f) Li, Y. X. Chin. J. Org. Chem. 2013, 33, 2334 (in Chinese).
(李元祥, 有机化学, 2013, 33, 2334.)
[4] (a) Chen, Y.; Tu, S. J.; Jiang, B.; Li, C. M. Chin. J. Org. Chem. 2007, 27, 1288 (in Chinese).
(陈艳, 屠树江, 姜波, 李春梅, 有机化学, 2007, 27, 1288.)
(b) Hua, G. P.; Xu, J. N.; Tu, S. J.; Wang, Q.; Zhang, J. P.; Zhu, X. T.; Li, T. J.; Zhu, S. L.; Zhang, X. J. Chin. J. Org. Chem. 2005, 25, 1610 (in Chinese).
(华国平, 徐佳宁, 屠树江,王倩, 张金鹏, 朱晓彤, 李团结, 朱松磊, 章晓镜, 有机化学, 2005, 25, 1610.)
(c) Liu, J.; Liu, G.; Tian, X. H.; Cao, L. H. Chin. J. Org. Chem. 2008, 28, 73 (in Chinese).
(刘俊, 刘罡, 田晓红, 曹玲华, 有机化学, 2008, 28, 73.)
(d) Shi, D. Q.; Shi, J. W.; Yao, H.; Jiang, H.; Wang, X. S. Chin. J. Org. Chem. 2008, 28, 261 (in Chinese).
(史达清, 拾景文, 姚浩, 蒋虹, 王香善, 有机化学, 2008, 28, 261.)
[5] (a) Wang, X. S.; Zhang, M. M.; Zeng, Z. S.; Shi, D. Q.; Tu, S. J.; Wei, X. Y.; Zong, Z. M. Tetrahedron Lett. 2005, 46, 7169.
(b) Peng, J.; Hao, W.; Wang, X.; Tu, S. J.; Ma, N.; Zhang, G. Chin. J. Chem. 2009, 27, 1707.
(c) Zhou, Y. J.; Chen, D. S.; Li, Y. L.; Liu, Y.; Wang, X. S. ACS Comb. Sci. 2013, 15, 498.
(d) Shi, F.; Yan, S.; Zhou, D.; Tu, S.; Zou, X.; Hao, W.; Zhang, X.; Han, Z.; Wu, S.; Cao, X. J. Heterocycl. Chem. 2009, 46, 563.
[6] (a) Chen, D. S.; Zhou, Y. J.; Li, Y. L.; Yao, C. S.; Wang, X. S. Comb. Chem. High Throughput Screening 2013, 16, 550.
(b) Li, C.; Mu, X. Y.; Li, Y. L. Liu, Y.; Wang, X. S. ACS Comb. Sci. 2013, 15, 267.
(c) Zhang, M. M.; Wang, W.; Wang, X. S. J. Heterocycl. Chem. 2014, 51, E349.
(d) Lu, W. Q.; Zhuang, R.; Chen, D. S.; Wang, X. S. Polycyclic Aromat. Compd. 2014, 34, 606.
/
| 〈 |
|
〉 |