

含苯环(E)-β-Farnesene类似物的设计、合成及驱避活性研究
收稿日期: 2016-03-14
修回日期: 2016-04-07
网络出版日期: 2016-04-20
基金资助
国家重点自然科学基金(No. 21132003)资助项目.
Design, Synthesis and Repellent Activity of (E)-β-Farnesene Analogues Containing Benzene Ring with Different Substitutions
Received date: 2016-03-14
Revised date: 2016-04-07
Online published: 2016-04-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 21132003).
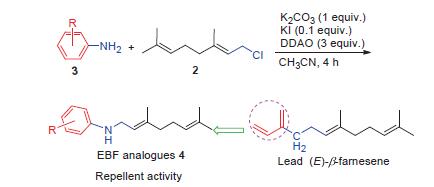
为了发现驱避蚜虫的新型高活性化合物,以蚜虫报警信息素(E)-β-farnesene(EBF)为先导,引入不同取代的苯环替代EBF结构中不稳定的共轭双键,设计了一系列含苯环的EBF类似物. 优化了一条苯胺和香叶基氯反应的N-烷基化路线,在最优条件下以39%~83%的收率合成了19个目标化合物,所有化合物结构均通过1H NMR、13C NMR及HRMS 确证. 生物活性测试结果表明,部分化合物对桃蚜具有一定驱避活性,其中4d、4h和4k驱避率分别高达56.3%、58.3%和50.3%. 目标化合物构效关系显示4-位卤素取代化合物活性较高.
张景朋 , 秦耀果 , 李文浩 , 凌云 , 杨立波 , 宋敦伦 , 杨新玲 . 含苯环(E)-β-Farnesene类似物的设计、合成及驱避活性研究[J]. 有机化学, 2016 , 36(8) : 1883 -1889 . DOI: 10.6023/cjoc201603021
In order to discover novel compounds with high-activity to control aphid, aphid alarm pheromone (E)-β-farnesene (EBF) was chosen as lead compound and a series of substituted EBF analogues were designed by replacing unstable conjugated double bond of EBF with different substitutions benzene ring. An N-alkylation route was optimized by using aniline and (E)-1-chloro-3,7-dimethyl-2,6-octadiene. Nineteen EBF analogues were synthesized with yields of 39%~83% in this optimization reaction condition. Their structures were confirmed by 1H NMR, 13C NMR and HRMS analysis. Bioassay showed that the title compounds demonstrated repellent activity against Myzus persicae (Sulzer). In particular, 4d, 4h and 4k exhibited excellent repellent activity of 56.3%, 58.3% and 50.3% respectively. The performed structure-activity relationship indicated that introduction of halogen group at the 4 position of phenyl ring displayed relatively higher activity.

[1] Wang, G.-P.; Yu, X.-D.; Fan, J.; Wang, C.-S.; Xia, L.-Q. J. Integr. Plant. Biol. 2015, 57, 770.
[2] Bruce, T. J. A.; Birkett, M. A.; Blande, J.; Hooper, A. M.; Martin, J. L.; Khambay, B.; Prosser, I.; Smart, L. E.; Wadhams, L. J. Pest. Manag. Sci. 2005, 61, 1115.
[3] Kang, T.-N.; Ling, Y.; Rui, C.-H.; Yang, X.-L.; Fan, X.-L.; Chen, F.-H. Chin. J. Org. Chem. 2008, 28, 617 (in Chinese).
(康铁牛, 凌云, 芮昌辉, 杨新玲, 范贤林, 陈馥衡, 有机化学, 2008, 28, 617.)
[4] Dahl, M. L. Dtsch. Ent. Z. 1971, 18, 121.
[5] Kislow, C. J.; Edward, L. J. Nature 1972, 235, 108.
[6] Bowers, W. S.; Nault, L. R.; Webb, R. E.; Dutky, S. R. Science 1972, 177, 1121.
[7] Mauchamp, B.; Picker, J. J. Agronomie 1987, 7, 523.
[8] Kunert, G.; Otto, S.; Rose, U. R.; Gershenzon, J.; Weisser, W. W. Ecol. Lett. 2005, 8, 596.
[9] Joachim, C.; Weisser, W. W. J. Chem. Ecol. 2015, 47, 267.
[10] Francis, F.; Lognay, G.; Haubruge, E. J. Chem. Ecol. 2004, 30, 741.
[11] Hatano, E.; Kunert, G.; Weisser, W. W. Eur. J. Entomol. 2008, 105, 797.
[12] Heuskin, S.; Lorge, S.; Godin, B.; Leroy, P.; Frere, I.; Verheggen, F. J.; Haubruge, E.; Wathelet, J. P.; Mestdagh, M.; Hance, T.; Lognay, G.; Pest. Manage. Sci. 2012, 68, 127.
[13] Joachim, C.; Hatano, E.; David, A.; Kunert, M.; Linse, C.; Weisser, W. W. J. Chem. Ecol. 2013, 39, 773.
[14] Griffiths, D. C.; Pickett, J. A. Entomol. Exp. Appl. 1980, 27, 199.
[15] Dawson, G. W.; Giffiths, D. C.; Pickett, J. A.; Plumb, R. T.; Woodcock, C. M.; Zhang, Z.-N. Pestic. Sci. 1988, 22, l7.
[16] Bowers, W. S.; Nishino, C.; Montgomery, M. E.; Nault, L. R. Insects Physiol. 1977, 23, 697.
[17] Sun, L.; Ling, Y.; Wang. C.; Sun, Y.-F.; Rui, C.-H.; Yang, X.-L. Chin. J. Org. Chem. 2011, 31, 2061 (in Chinese).
(孙亮, 凌云, 王灿, 孙玉凤, 芮昌辉, 杨新玲, 有机化学, 2011, 31, 2061.)
[18] Qin, Y.-G.; Qu, Y.-Y.; Zhang, J.-P.; Tan, X.-Q.; Song, L.-F.; Li, W.-H.; Song, D.-L.; Yang, X.-L. Chin. J. Org. Chem. 2015, 35, 455 (in Chinese).
(秦耀果, 曲焱焱, 张景朋, 谭晓庆, 宋丽芳, 李文浩, 宋敦伦, 杨新玲, 有机化学, 2015, 35, 455.)
[19] Sun, Y.-F.; Li, Y.-Q.; Ling, Y.; Yu, H.-L.; Yang, S.-X.; Yang, X.-L. Chin. J. Org. Chem. 2011, 31, 1425 (in Chinese).
(孙玉凤, 李永强, 凌云, 宇红莲, 杨绍祥, 杨新玲, 有机化学, 2011, 31, 1425.)
[20] Sun, Y.-F.; Filomena, D.; Qiao, H.-L.; Immacolata, I.; Yang, S.-X.; Ling, Y.; Lea, R.; Donatella, B.; Patrizia, F.; Yang, X.-L.; Paolo, P. PLoS One 2012, 7, e32759.
[21] Sun, Y.-F.; Qiao, H.-L.; Ling, Y.; Yang, S.-X.; Rui, C.-H.; Paolo Pelosi; Yang, X.-L. J. Agric. Food Chem. 2011, 59, 2456.
[22] Ohshima, T,; Miyamoto, Y,; Ipposhi, J.; Nakahara, Y.; Utsunomiya, M.; Mashima, K. J. Am. Chem. Soc. 2009, 131, 14317.
[23] Zhao, Y.-S.; Foo, S. W.; Saito, S. Angew. Chem., Int. Ed. 2011, 50, 3006.
[24] Kazuhide, T.; Tsuneaki, Y.; Susumu, A.; Hidenori, K.; Takanao, T.; Hidemasa, T.; Akira, M.; Ryoji, N.; Sei, O. J. Am. Chem. Soc. 1984, 106, 5208.
[25] Shi, H.; Lu, F.; Xiong, J.-J.; Xu, Y.-X.; Wang, J. Fine Chem. Intermed. 2008, 38, 8 (in Chinese).
(师华, 陆峰, 熊家锦, 徐永祥, 王菁, 精细化工中间体, 2008, 38, 8.)
[26] Peat, A. J.; Buchwald, S. L. J. Am. Chem. Soc. 1996, 118, 1028.
[27] Romera, J. L.; Cid, J. M.; Trabanco, A. A. Tetrahedron Lett. 2004, 45, 8797.
[28] Siswanto, C.; Rathman, J. F. J. Colloid Interface Sci. 1997, 196, 99.
[29] Pinchin, R.; De Oliveira Filho, A. M.; Figueiredo, M. J.; Muller, C. A.; Gilbert, B.; Szumlewicz, A. P.; Benson, W. W. J. Econ. Entomol. 1978, 71, 950.
[30] Sun, L. Ph.D. Dissertation, China Agricultural University, Beijing, 2013 (in Chinese).
/
| 〈 |
|
〉 |