

磷亲核试剂对缺电子烯烃不对称共轭加成反应的进展
收稿日期: 2016-02-19
修回日期: 2016-04-20
网络出版日期: 2016-04-29
基金资助
国家自然科学基金(Nos. 20902099,21172238,21472218)资助项目.
Recent Advances in the Asymmetric Conjugate Addition Reactions of Phosphorus Nucleophiles to Electron-Deficient Alkenes
Received date: 2016-02-19
Revised date: 2016-04-20
Online published: 2016-04-29
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20902099, 21172238, 21472218).
李振 , 段伟良 . 磷亲核试剂对缺电子烯烃不对称共轭加成反应的进展[J]. 有机化学, 2016 , 36(8) : 1805 -1813 . DOI: 10.6023/cjoc201602018
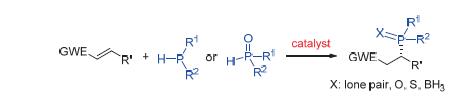
Chiral phosphines are very useful in the fields of asymmetric catalysis, pharmaceutical chemistry, and materials. These compounds have been used as ligands coordinated to transition metals or organocatalysts in various reactions to furnish optically active products with high efficiency. Asymmetric phosphorus-addition reaction can be utilized for the direct preparation of chiral phosphorus compounds. This review summarizes the recent progress in the field of enantioselective phosphorus-addition reactions. Several examples of transition metal- and organocatalyst-promoted asymmetric hydrophosphination reactions of electron-deficient alkenes have been introduced.
[1] For reviews on metal-catalyzed asymmetric 1,4-addition, see:
(a) Hayashi, T.; Yamasaki, K. Chem. Rev. 2003, 103, 2829.
(b) Miyaura, N. Synlett 2009, 2039.
(c) Christoffers, J.; Koripelly, G.; Rosiak, A.; Rössle, M. Synthesis 2007, 1279.
For reviews on organocatalytic asymmetric 1,4-addition, see:
(d) Tsogoeva, S. B. Eur. J. Org. Chem. 2007, 1701.
(e) Vicario, J. L.; Badía, D.; Carrillo, L. Synthesis 2007, 2065.
(f) Enders, D.; Wang, C.; Liebich, J. X. Chem.-Eur. J. 2009, 15, 11058.
(g) Enders, D.; Lüttgen, K.; Narine, A. A. Synthesis 2007, 959.
(h) Nising, C. F.; Bräse, S. Chem. Soc. Rev. 2008, 37, 1218.
(i) Liu, Q.; Tian, P.; Tong, X.; Lin, G.-Q. Chin. J. Org. Chem. 2015, 35, 1 (in Chinese).
(刘强, 田平, 童晓峰, 林国强, 有机化学, 2015, 35, 1.)
(j) For a review of 1,4-addition reaction of phosphorus nucleophiles to electron-deficient olefins, see: Enders, D.; Saint-Dizier, A.; Lannou, M.-I.; Lenzen, A. Eur. J. Org. Chem. 2006, 29.
[2] (a) Kovacik, I.; Wicht, D. K.; Grewal, N. S.; Glueck, D. S.; Incarvito, C. D.; Guzei, I. A.; Rheingold, A. L. Organometallics 2000, 19, 950.
(b) Scriban, C.; Kovacik, I.; Glueck, D. S. Organometallics 2005, 24, 4871.
[3] For reviews on catalytic asymmetric synthesis of chiral phosphanes, see: (a) Glueck, D. S. Synlett 2007, 2627.
(b) Glueck, D. S. Chem.-Eur. J. 2008, 14, 7108.
(c) Harvey, J. S. Gouverneur, V. Chem. Commun. 2010, 7477.
(d) Zhao, D.; Wang, R. Chem. Soc. Rev. 2012, 41, 2095.
(e) Yao, Q.; Wang, A.; Pu, J.; Tang, Y. Chin. J. Org. Chem. 2014, 34, 292 (in Chinese).
(姚秋丽, 王安俊, 蒲家志, 唐瑜敏, 有机化学, 2014, 34, 292.)
[4] (a) Bansal, R. K. Phosphorus Heterocycles II (Topics in Heterocyclic Chemistry, Vol. 21), Springer, Berlin, 2010.
(b) Börner, A. Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Applications, Wiley-VCH, Weinheim, 2008, Vols. 1~3.
(c) Peruzzini, M.; Gonsalvi, L. Phosphorus Compounds: Advanced Tools in Catalysis and Material Sciences, Springer, Berlin, 2011.
(d) Xu, Q.; Zhao, C.; Zhou, Y.; Yin, S.; Han, Li. Chin. J. Org. Chem. 2012, 32, 1761 (in Chinese).
(徐清, 赵长秋, 周永波, 尹双凤, 韩立彪, 有机化学, 2012, 32, 1761.)
[5] (a) Sadow, A. D.; Haller, I.; Fadini, L.; Togni, A. J. Am. Chem. Soc. 2004, 126, 14704.
(b) Sadow, A. D.; Togni, A. J. Am. Chem. Soc. 2005, 127, 17012.
[6] Bartoli, G.; Bosco, M.; Carlone, A.; Locatelli, M.; Mazzanti, A.; Sambri, L.; Melchiorre, P. Chem. Commun. 2007, 722.
[7] (a) Carlone, A.; Bartoli, G.; Bosco, M.; Sambri, L.; Melchiorre, P. Angew. Chem., Int. Ed. 2007, 46, 4504.
(b) Ibrahem, I.; Rios, R.; Vesely, J.; Hammar, P.; Eriksson, L.; Himo, F.; Cordova, A. Angew. Chem., Int. Ed. 2007, 46, 4507.
(c) Ibrahem, I.; Hammar, P.; Vesely, J.; Rios, R.; Eriksson, L.; Cordova, A. Adv. Synth. Catal. 2008, 350, 1875.
[8] Feng, J.-J.; Chen, X.-F.; Shi, M.; Duan, W.-L. J. Am. Chem. Soc. 2010, 132, 5562.
[9] For reviews on pincer metal complexes, see: (a) Albrecht, M.; van Koten, G. Angew. Chem., Int. Ed. 2001, 40, 3750.
(b) Selander, N.; Szabó, K. J. Chem. Rev. 2011, 111, 2048.
(c) Hao, X.; Niu, J.; Zhao, X.; Gong, J.; Song, M. Chin. J. Org. Chem. 2013, 33, 663 (in Chinese).
(郝新奇, 牛俊龙, 赵雪梅, 龚军芳, 宋毛平, 有机化学, 2013, 33, 663.)
For synthesis and application of the chiral PCP-PdCl complex, see: (d) Longmire, J. M.; Zhang, X. Tetrahedron Lett. 1997, 38, 1725.
(e) Longmire, J. M.; Zhang, X.; Shang, M. Organometallics 1998, 17, 4374.
[10] (a) Du, D.; Duan, W.-L. Chem. Commun. 2011, 47, 11101.
(b) Chen, Y.-R.; Duan, W.-L. Org. Lett. 2011, 13, 5824.
(c) Feng, J.-J.; Huang, M.; Lin, Z.-Q.; Duan, W.-L. Adv. Synth. Catal. 2012, 354, 3122.
(d) Du, D.; Lin, Z.-Q.; Lu, J.-Z.; Li, C.; Duan, W.-L. Asian J. Org. Chem. 2013, 2, 392.
(e) Lu, J.; Ye, J.; Duan, W.-L. Org. Lett. 2013, 15, 5016.
(f) Huang, M.; Li, C.; Duan, W.-L.; Xu, S. Chem. Commun. 2012, 48, 11148.
[11] Huang, Y.; Pullarkat, S. A.; Li, L.; Leung, P.-H. Chem. Commun. 2010, 6950.
[12] (a) Huang, Y.; Chew, R. J.; Li, Y.; Pullarkat, S. A.; Leung, P.-H. Org. Lett. 2011, 13. 5862.
(b) Huang, Y.; Pullarkat, S. A.; Teong, S.; Chew, R. J.; Li, Y.; Leung, P.-H. Organometallics 2012, 31, 4871.
(c) Chew, R. J.; Huang, Y.; Li, Y.; Pullarkat, S. A.; Leung, P.-H. Adv. Synth. Catal. 2013, 355, 1403.
(d) Chew, R. J.; Teo, K. Y.; Huang, Y.; Li, B.-B.; Li, Y.; Pullarkat, S. A.; Leung, P.-H. Chem. Commun. 2014, 50, 8768.
(e) Chew, R. J.; Lu, Y.; Jia, Y.-X.; Li, B.-B.; Wong, E. H. Y.; Goh, R.; Li, Y.; Huang, Y.; Pullarkat, S. A.; Leung, P.-H. Chem.-Eur. J. 2014, 20, 14514.
(f) Chew, R. J.; Sepp, K.; Li, B.-B.; Li, Y.; Zhu, P.-C.; Tan, N. S.; Leung, P.-H. Adv. Synth. Catal. 2015, 357, 3297.
[13] (a) Yang, X.-Y.; Tay, W. S.; Li, Y.; Pullarkat, S. A.; Leung, P.-H. Organometallics 2015, 34, 5196.
(b) Yang, X.-Y.; Tay, W.; Li, Y.; Pullarkat, S.; Leung, P.-H. Chem. Commun. 2016, 52, 4211.
[14] (a) Yang, M.-J.; Liu, Y.-J.; Gong, J.-F.; Song, M.-P. Organometallics 2011, 30, 3793.
(b) Hao, X.-Q.; Zhao, Y.-W.; Yang, J.-J.; Niu, J.-L.; Gong, J.-F.; Song, M.-P. Organometallics 2014, 33, 1801.
(c) Hao, X. Q.; Huang, J. J.; Wang, T.; Lv, J.; Gong, J. F.; Song, M. P. J. Org. Chem. 2014, 79, 9512.
[15] (a) Ding, B.; Zhang, Z.; Xu, Y.; Liu, Y.; Sugiya, M.; Imamoto, T.; Zhang, W. Org. Lett. 2013, 15, 5476.
(b) Xu, Y.; Yang, Z.; Ding, B.; Liu, D.; Liu, Y.; Sugiya, M.; Imamoto, T.; Zhang, W. Tetrahedron 2015, 71, 6832.
[16] For reviews on asymmetric 1,6-addition reactions, see:
(a) Csákÿ, A. G.; la Herran, G. de.; Murcia, M. C. Chem. Soc. Rev. 2010, 39, 4080.
(b) Biju, A. T. ChemCatChem 2011, 3, 1847.
(c) Silva, E. M. P.; Silva, A. M. S. Synthesis 2012, 44, 3109.
[17] (a) Lu, J.; Ye, J.; Duan, W.-L. Chem. Commun. 2014, 50, 698.
(b) Huang, J.; Zhao, M.-X.; Duan, W.-L. Tetrahedron Lett. 2014, 55, 629.
[18] Huang, Y.; Pullarkat, S. A.; Li, Y.; Leung, P.-H. Inorg. Chem. 2012, 51, 2533.
[19] Li, C.; Li, W.-X.; Xu, S.; Duan, W.-L. Org. Chem. Front. 2014, 1, 541.
[20] Chen, Y.-R.; Feng, J.-J.; Duan, W.-L. Tetrahedron Lett. 2014, 55, 595.
[21] Fu, X.; Jiang, Z.; Tan, C.-H. Chem. Commun. 2007, 5058.
[22] (a) Zhao, D.; Yuan, Y.; Chan, Albert S. C.; Wang, R. Chem.-Eur. J. 2009, 15, 2738.
(b) Zhao, D.; Wang, Y.; Mao, L.; Wang. R. Chem.-Eur. J. 2009, 15, 10983.
(c) Zhao, D.; Mao, L.; Wang, Y.; Yang, D.; Zhang, Q.; Wang, R. Org. Lett. 2010, 12, 1880.
(d) Zhao, D.; Mao, L.; Yang, D.; Wang, R. J. Org. Chem. 2010, 75, 6756.
(e) Zhao, D.; Mao, L.; Wang, L.; Yang, D.; Wang. R. Chem. Commun. 2012, 48, 889.
(f) Zhao, D.; Wang, L.; Yang, D.; Zhang, Y.; Wang. R. Chem.-Eur. J. 2012, 7, 881.
[23] Wen, S.; Li, L.; Wu, H.; Yu, F.; Liang, X.; Ye, J. Chem. Commun. 2010, 4806.
[24] Russo, A.; Lattanzi, A. Eur. J. Org. Chem. 2010, 6736.
[25] Luo, X.; Zhou, Z.; Li, X.; Liang, X.; Ye, J. RSC Adv. 2011, 1, 698.
[26] Hatano, M.; Horibe, T.; Ishihara, K. Angew. Chem., Int. Ed. 2013, 52, 4549.
[27] Geng, Z-C.; Zhang, J.-X.; Li, N.; Chen, J.; Huang, X.-F.; Zhang, S.-Y.; Li, H.-Y.; Tao, J.-C.; Wang, X.-W. Tetrahedron 2014, 70, 417.
/
| 〈 |
|
〉 |