

芳香化合物亲核、亲电反应活性的理论预测和实验反应速率的相关性研究
收稿日期: 2016-02-26
修回日期: 2016-05-12
网络出版日期: 2016-06-01
基金资助
国家自然科学基金(Nos.21173020,21473008)资助项目.
Theoretical Study on the Correlation of the Experimental Nucleophilic and Electrophilic Reaction Rates of Aromatic Compounds with the Prediction Results of Theoretical Methods
Received date: 2016-02-26
Revised date: 2016-05-12
Online published: 2016-06-01
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21173020, 21473008).
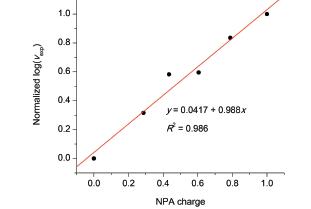
选择自然布局分析(NPA)电荷、Hirshfeld电荷、分子范德华表面1.6Å处的静电势、最低未占据轨道(LUMO)成分、分子范德华表面1.6Å处的平均局部离子化能、简缩福井函数和简缩双描述符来预测芳香族化合物亲核加成、亲核取代和亲电反应的活性位点,并分析理论预测结果和实验反应速率之间的相关性.发现这些方法都能准确地预测反应的活性位点.还发现不管对亲核反应还是对亲电反应,体现局部电子硬度的方法,如Hirshfeld电荷和分子范德华表面1.6Å处的静电势,其预测结果的大小能较好地反映实验反应速率的相对大小,而体现局部电子软度的方法,简缩福井函数和简缩双描述符,其预测结果和实验反应速率的相关性较小.
曹静思 , 陈飞武 . 芳香化合物亲核、亲电反应活性的理论预测和实验反应速率的相关性研究[J]. 有机化学, 2016 , 36(10) : 2463 -2471 . DOI: 10.6023/cjoc201602026
Natural population analysis (NPA) charge, Hirshfeld charge, electrostatic potential, average local ionization energy, orbital composition of lowest unoccupied molecular orbital (LUMO), condensed Fukui function and condensed dual descriptor were exploited to predict the reaction active sites of nucleophilic and electrophilic reactions of aromatic compounds. It was found that the predicted reaction sites of these methods were all in consistent with the experimental results. It was also found that the correlations of the prediction results of theoretical methods reflecting local hardness such as Hirshfeld charges and electrostatic potential with the experimental reaction rate were excellent no matter the reactions of aromatic compounds are nucleophilic or electrophilic. However, the prediction results of theoretical methods reflecting local softness such as the condensed Fukui function and the condensed dual descriptor were in poor correlation with the experimental reaction rates as unexpected.

[1] Wade, L. G. Organic Chemistry, 6th ed., Pearson, London, 2006.
[2] Cao, S.; Jing, Y. F.; Liu, Y. Y.; Wan, J. P. J. Org. Chem. 2014, 34, 876(in Chinese). (曹硕, 景艳锋, 刘云云, 万结平, 有机化学, 2014, 34, 876.)
[3] Shen, S. S. Chin. J. Org. Chem. 2014, 34, 2448(in Chinese). (沈舒苏, 有机化学, 2014, 34, 2448.)
[4] Esteves, P. M.; Carneiro, J. W. de M.; Cardoso, S. P.; Barbosa, A. G. H.; Laali, K. K.; Rasul, G.; Prakash, G. K. S.; Oláh, G. A. J. Am. Chem. Soc. 2003, 125, 4836.
[5] Hänggi, P.; Talkner, P.; Borkovec, M. Rev. Mod. Phys. 1990, 62, 251.
[6] Zhang, J. Z. H. Theory and Application of Quantum Molecular Dynamics, World Scientific, Singapore, 1999.
[7] Lu, T.; Chen, F. W. Acta Chim. Sin. 2011, 69, 2393(in Chinese). (卢天, 陈飞武, 化学学报, 2011, 69, 2393.)
[8] Murray, J. S.; Politzer, P. WIREs Comput. Mol. Sci. 2011, 1, 153.
[9] Xu, H. Y.; Wang, W.; Zhou, W. J. Acta Chim. Sinica 2013, 71, 1175(in Chinese). (许惠英, 王维, 邹建卫, 化学学报, 2013, 71, 1175.)
[10] Lu, T.; Chen, F. W. J. Mol. Model 2013, 19, 5387.
[11] Zhang, Q.; Wang, Y.; Liu, C.; Yang, Z. Z. Acta Chim. Sinica 2014, 72, 956(in Chinese). (张千慧, 王阳, 刘翠, 杨忠志, 化学学报, 2014, 72, 956.)
[12] Liu, S. B.; Rong, C.; Lu, T. J. Phys. Chem. A 2014, 118, 3698.
[13] Zhou, X. Y.; Rong, C. Y.; Lu, T.; Liu, S. B. Acta Phys.-Chim. Sin. 2014, 30, 2055(in Chinese). (周夏禹, 荣春英, 卢天, 刘述斌, 物理化学学报, 2014, 30, 2055.)
[14] Wu, W. J.; Wu, Z. M.; Rong, C. Y.; Lu, T.; Huang, Y.; Liu, S. B. J. Phys. Chem. A 2015, 119, 8216.
[15] Wu, Z. M.; Rong, C. Y.; Lu, T.; Ayer, P. W.; Liu, S. B. Phys. Chem. Chem. Phys. 2015, 17, 27052.
[16] Liu, S. B. Acta Phys.-Chim. Sin. 2016, 32, 98.
[17] Fu, R.; Lu, T.; Chen, F. W. Acta Phys.-Chim. Sin. 2014, 30, 628(in Chinese). (付蓉, 卢天, 陈飞武, 物理化学学报, 2014, 30, 628.)
[18] Cao, J. S.; Ren, Q.; Chen, F. W.; Lu, T. Sci. China Chem. 2015, 58, 1845.
[19] Ammer, J.; Nolte, C.; Mayr, H. J. Am. Chem. Soc. 2012, 134, 13902.
[20] Horn, M.; Schappele, L. H.; Lang-Wittkowski, G.; Mayr, H.; Ofial, A. R. Chem.-Eur. J. 2013, 19, 249.
[21] Shi, L.; Chu, Y.; Knochel, P.; Mayr, H. Angew. Chem., Int. Ed. 2008, 47, 202.
[22] March, J. Advanced Organic Chemistry:Reactions, Mechanisms and Structure, Vol. 4, Wiley-Interscience Publication, United States of America, 1992, pp. 505~510.
[23] Lakhdar, S.; Westermaier, M.; Terrier, F.; Goumont, R.; Boubaker, T.; Ofial, A. R.; Mayr, H. J. Org. Chem. 2006, 71, 9088.
[24] Westermaier, M.; Mayr, H. Org. Lett. 2006, 8, 4791.
[25] Kuivila, H. G.; Hendrickson, A. R. J. Am. Chem. Soc. 1952, 74, 5068.
[26] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009.
[27] Axel, D.; Becke J. Chem. Phys. 1993, 98, 1372.
[28] Hariharan, P. C.; Pople, J. A. Theor. Chim. Acta 1973, 28, 213.
[29] Lu, T.; Chen, F. W. J. Comput. Chem. 2012, 33, 580.
[30] Hirshfeld, F. L. Theor. Chim. Acta 1977, 44, 129.
[31] Alan, R. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83(2), 15.
[32] Glendening, E. D.; Landis, C. R.; Weinhold, F. WIREs Comput. Mol. Sci. 2012, 2, 1.
[33] Nalewajski; Parr, R. F. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 8879.
[34] Parr, R. G.; Yang, W. Density Functional Theory of Atoms and Molecules, Springer, Netherlands, 1980.
[35] Parr, R. G.; Donnelly, R. A.; Levy, M.; Palke, W. E. J. Chem. Phys. 1978, 68, 3801.
[36] Liu, S. B. Acta Phys.-Chim. Sin. 2009, 25, 590.
[37] Geerlings, P.; Proft, De F.; Langenaeker, W. Chem. Rev. 2003, 103, 1793.
[38] Yang, W.; Mortier, W. J. J. Am. Chem. Soc. 1986, 108, 5708.
[39] Jin, J. L.; Li, H. B.; Lu, T.; Duan, Y. A.; Geng, Y.; Wu, Y.; Su, Z. M. J. Mol. Model. 2013, 19, 3437.
[40] Chattaraj, P. K.; Maiti, B.; Sarkar, U. J. Phys. Chem. A 2003, 107, 4973.
[41] Oláh, J.; Van Alsenoy, C.; Sannigrahi, A. B. J. Phys. Chem. A 2002, 106, 3885.
[42] Lu, T.; Chen, F. W. Acta Phys.-Chim. Sin. 2012, 28, 1(in Chinese). (卢天, 陈飞武, 物理化学学报, 2012, 28, 1.)
[43] Politzer, P.; Murray, J. S. In Reviews in Computational Chemistry, Vol. 2, Eds.:Lipkowitz, K. B.; Boyd, D. B., Wiley, New York, 1991, p. 273.
[44] Politzer, P.; Murray, J. S. In Chemical Reactivity Theory:A Density Functional View, Ed.:Chattaraj, P. K., CRC Press, London, 2009, p. 243.
[45] Geerlings, P.; Langenaeker, W.; Proft, D. F.; Baeten, A. Theor. Comput. Chem. 1996, 3, 587.
[46] Politzer, P.; Murray, J. S.; Concha, M. C. Int. J. Quantum Chem. 2002, 88, 19.
[47] Politzer, P.; Laurence, P. R.; Jayasuriya, K. Environ. Health Perspect. 1985, 61, 191.
[48] Sjoberg, P.; Politzer, P. J. Phys. Chem. 1990, 94, 3959.
[49] Bader, R. F. W.; Carroll, M. T.; Cheeseman, J. R.; Chang, C. J. Am. Chem. Soc. 1987, 109, 7968.
[50] Lu, T.; Chen, F. W. J. Mol. Graphics Modell. 2012, 38, 314.
[51] Murray, J. S.; Peralta-Inga, Z.; Politzer, P.; Ekanayake, K.; LeBreton, P. Int. J. Quantum Chem. 2001, 83, 245.
[52] Sjoberg, P.; Murray, J. S.; Brinck, T.; Politzer, P. Can. J. Chem. 1990, 68, 1440.
[53] Politzer, P.; Murray, J. S. In Theoretical Aspects of Chemical Reactivity, Ed.:Toro-Labbé, A., Elsevier, Amsterdam, 2007, p. 119.
[54] Fukui, K. Theory of Orientation and Stereoselection, Springer, Berlin, 2013.
[55] Fukui, K.; Yonezawa, T.; Shingu, H. J. Chem. Phys. 1952, 20, 722.
/
| 〈 |
|
〉 |