

新型含1,3,4-噁二唑环结构的氰基丙烯酸酯类化合物的合成及生物活性研究
收稿日期: 2016-05-17
修回日期: 2016-06-06
网络出版日期: 2016-06-20
基金资助
国家自然科学基金(No.21372135)、江苏省“六大人才高峰”(No.2013-SWYY-013)和南通市科技计划(Nos.AS2014011,CP12013002,MS22015020)资助项目.
Synthesis and Biological Activities of Novel Cyanoacrylates Containing 1,3,4-Oxadiazole Moiety
Received date: 2016-05-17
Revised date: 2016-06-06
Online published: 2016-06-20
Supported by
Project supported by the National Natural Science Foundation of China (No.21372135), the Research Foundation of the Six People Peak of Jiangsu Province (No.2013-SWYY-013), and the Science and Technology Project Fund of Nantong City (Nos.AS2014011, CP12013002, MS22015020).
石玉军 , 李阳 , 方源 , 陈佳 , 叶林玉 , 葛书山 , 戴红 . 新型含1,3,4-噁二唑环结构的氰基丙烯酸酯类化合物的合成及生物活性研究[J]. 有机化学, 2016 , 36(10) : 2472 -2478 . DOI: 10.6023/cjoc201605029
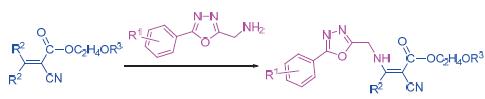
In order to find new cyanoacrylate lead compounds, a series of novel cyanoacrylates carrying 1,3,4-oxadiazole moiety were prepared by the method of active substructure combination. The structures of the title compounds were characterized by 1H NMR, 13C NMR and elemental analysis. Preliminary bioassay data displayed that in postemergence treatment compound 8b had 80% herbicidal activity against Stellaria media at 1500 g/ha and compound 8j showed 70% herbicidal activity against Chenopodium serotinum L. at 1500 g/ha. Additionally, compounds 8e, 8k, 8m and 8n exhibited good anti-tumor activity against HepG2 cells with the IC50values of 8.2, 5.9, 8.0 and 12.6 μmol/L, respectively.
Key words: 1,3,4-oxadiazole; 2-cyanoacrylates; synthesis; biological activity
[1] Holla, B. S.; Gonsalves, R.; Shenoy, S. Eur. J. Med. Chem. 2000, 35, 267.
[2] Chen, H. S.; Li, Z. M.; Li, J. F. Chem. J. Chin. Univ. 2000, 21, 1520(in Chinese). (陈寒松, 李正名, 李佳凤, 高等学校化学学报, 2000, 21, 1520.)
[3] Zou, X. J.; Lai, L. H.; Jin, G. Y.; Zhang, Z. X. J. Agric. Food Chem. 2002, 50, 3757.
[4] Chen, W.; Chen, Q.; Wu, Q. Y.; Yang, G. F. Chin. J. Org. Chem. 2005, 25, 1477(in Chinese). (陈悟, 陈琼, 吴琼友, 杨光富, 有机化学, 2005, 25, 1477.)
[5] Long, D. Q.; Li, D. J.; Cai, W. Q.; Chen, S. S. Chin. Chem. Lett. 2006, 17, 1427.
[6] Zhu, Y. Q.; Liu, W. M.; Liu, B.; Hu, F. Z.; Zhu, R.; Zou, X. M.; Yang, H. Z. Chin. J. Org. Chem. 2009, 29, 638(in Chinese). (朱有全, 刘卫敏, 刘斌, 胡方中, 朱然, 邹小毛, 杨华铮, 有机化学, 2009, 29, 638.)
[7] Yu, S. Q. Modern Agrochem. 2003, 2, 11(in Chinese). (于淑琴, 现代农药, 2003, 2, 11.)
[8] Zhu, J. R.; Jiang, Y. F. Chin. J. Pestic. 2015, 54, 400(in Chinese). (朱建荣, 姜友法, 农药, 2015, 54, 400.)
[9] Ruel, R.; Thibeault, C.; L'Heureux, A.; Martel, A.; Cai, Z. W.; Wei, D.; Qian, L. G.; Barrish, J. C.; Mathur, A.; D'Arienzo, C.; Hunt, J. T.; Kamath, A.; Marathe, P.; Zhang, Y. P.; Derbin, G.; Wautlet, B.; Mortillo, S.; Jeyaseelan, R.; Henley, B.; Tejwani, R.; Bhide, R. S.; Trainor, G. L.; Fargnolib, J.; Lombardob, L. J. Bioorg. Med. Chem. Lett. 2008, 18, 2985.
[10] Formagio, A. S. N.; Tonin, L. T. D.; Foglio, M. A.; Madjarof, C. Bioorg. Med. Chem. 2008, 16, 9660.
[11] Zhang, S.; Luo, Y.; He, L, Q.; Liu, Z. J.; Jiang, A. Q.; Yang, Y. H.; Zhu, H. L. Bioorg. Med. Chem. 2013, 21, 3723.
[12] Kumar, A.; D'Souza, S. S.; Nagaraj, S. R. M.; Gaonkar, S. L. Cancer Chemother. Pharmacol. 2009, 64, 1221.
[13] Dash, S.; Kumar, B. A.; Singh, J.; Maiti, B. C.; Maity, T. K. Med. Chem. Res. 2011, 20, 1206.
[14] Wang, L. G.; Wang, F. Y.; Diao, Y. M.; Ni, J. P.; Wei, P. Chin. J. Org. Chem. 2005, 25, 1254(in Chinese). (王龙根, 王凤云, 刁亚梅, 倪珏萍, 韦萍, 有机化学, 2005, 25, 1254.)
[15] Song, B. A.; Yang, S.; Zhong, H. M.; Jin, L. H.; Hu, D. Y.; Lin, G. J. Fluorine Chem. 2005, 126, 87.
[16] Zou, X. M.; Yu, L. M.; Gao, Y.; Shi, H. L.; Fei, J.; Liu, B.; Li, H. F.; Hu, F. Z.; Yang, H. Z. Chin. J. Org. Chem. 2006, 26, 337(in Chinese). (邹小毛, 郁丽敏, 高颍, 施欢乐, 裴江, 刘斌, 李慧芬, 胡方中, 杨华铮, 有机化学, 2006, 26, 337.)
[17] Zhang, H.; Li, X. Y.; Hu, D. Y.; Yang, S.; Fan, H. T.; Wei, X.; Song, B. A. Chin. J. Org. Chem. 2011, 31, 1419(in Chinese). (章浩, 李向阳, 胡德禹, 杨松, 范会涛, 宋宝安, 有机化学, 2011, 31, 1419.)
[18] Zhong, S. H.; Wang, C. F.; Song, Q. X.; Fan, M. L.; Liu, B. Y.; Wei, D. M.; Liu, J. B. Chin. J. Org. Chem. 2014, 34, 2324(in Chinese). (钟世华, 王春凤, 宋青霞, 范明亮, 刘兵玉, 危冬梅, 刘建兵, 有机化学, 2014, 34, 2324.)
[19] Wang, X.; Wang, C. Q.; Fu, C. R.; Zou, X. M. Chin. J. Org. Chem. 2015, 35, 92(in Chinese). (王鑫, 王朝强, 傅翠蓉, 邹小毛, 有机化学, 2015, 35, 92.)
[20] Zhang, H. P.; Song, B. A.; Zhong, H. M.; Yang, S.; Jin, H. L.; Hu, D. Y.; He, W. J. Heterocycl. Chem. 2005, 42, 1211.
[21] Wang, Q. M.; Sun, H. K.; Cao, H. Y.; Cheng, M. R.; Huang, R. Q. J. Agric. Food Chem. 2003, 51, 5030.
[22] Zhao, Q. Q.; Liu, S. H.; Li, Y. H.; Wang, Q. M. J. Agric. Food Chem. 2009, 57, 2849.
[23] Ouyang, G. P.; Song, B. A.; Zhang, H. P.; Yang, S.; Jin, L. H.; Li, Q. Z.; Hu, D. Y. Molecules 2005, 10, 1351.
[24] Yang, R. S.; Hu, G. Q.; Xie, S. Q.; Huang, W. L. J. Chin. Pharm. 2008, 43, 388(in Chinese). (杨锐生, 胡国强, 谢松强, 黄文龙, 中国药学杂志, 2008, 43, 388.)
[25] Liu, J. C.; Liu, Y. J.; He, H. W. Chin. J. Org. Chem. 2015, 35, 462(in Chinese). (刘建超, 刘勇军, 贺红武, 有机化学, 2015, 35, 462.)
[26] Song, B. A.; Yang, S.; Zhong, H. M.; Jin, L. H.; Hu, D. Y.; Liu, G. J. Fluorine Chem. 2015, 126, 87.
[27] Zheng, Q. Z.; Zhang, X. M.; Xu, Y.; Cheng, K.; Jiao, Q. C.; Zhu, H. L. Bioorg. Med. Chem. 2010, 18, 7836.
/
| 〈 |
|
〉 |