

硫酸铈介导下黄酮和2-芳基-4-喹啉酮衍生物的合成
收稿日期: 2016-03-22
修回日期: 2016-06-20
网络出版日期: 2016-07-08
基金资助
国家自然科学基金(No.31370370)、湖南省科技厅科技计划项目(No.2013SK5077)及海南省社会发展科技专项(No.SF201419)资助项目.
Ce(SO4)2·4H2O Mediated Synthesis of Flavones and 2-Phenyl-4-quinolones
Received date: 2016-03-22
Revised date: 2016-06-20
Online published: 2016-07-08
Supported by
Project supported by the National Natural Science Foundation of China (No.31370370),the Science and Technology Planning Project of Hunan Pprovince (No.2013SK5077) and the Social Development of Science and Technology Project of Hainan Province (No.SF201419)
刘瑞环 , 王绪礼 , 成飞 , 李福双 , 徐康平 , 谭桂山 . 硫酸铈介导下黄酮和2-芳基-4-喹啉酮衍生物的合成[J]. 有机化学, 2016 , 36(11) : 2677 -2682 . DOI: 10.6023/cjoc201603036
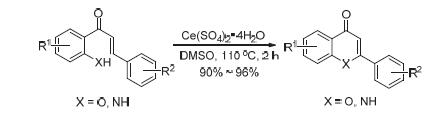
A simple and powerful procedure for the synthesis of flavones and quinolones was developed by using Ce(SO4)2·4H2O as oxidant, readily available 2'-hydroxychalcones and 2'-aminochalcones as raw materials, and dimethyl sulfoxide (DMSO) as solvent at 110℃. The new procedure is characterized with raw material easy to get, low manufacturing cost, mild reaction condi-tions and high yields.
Key words: Ce(SO4)2·; 4H2O; 2'-hydrochalcone; 2'-aminochalcone; flavone; quinolone
[1] Silva, A. M. S.; Pinto, D. C. G. A.; Cavaleiro, J. A. S. Tetrahedron Lett. 1994, 35, 5899.
[2] Akama, T.; Shida, Y.; Sugaya, T.; Ishida, H.; Gomi, K.; Kasai, M. J. Med. Chem. 1996, 39, 3461.
[3] Alam, S.; Sarkar, Z.; Islam, A. J. Chem. Sci. 2004, 116, 29.
[4] Hans, N.; Grover, S. K. Synth. Commun. 1993, 23, 1021.
[5] Donnelly, J. A.; Farrell, D. F. J. Org. Chem. 1990, 55, 1757.
[6] Ahmed, N.; Ali, H.; van Lier, J. E. Tetrahedron Lett. 2005, 46, 253.
[7] Jung, S-H.; Cho, S-H.; Dang, T. H.; Lee, J-H.; Ju, J-H.; Kim, M-K.; Lee, S-H.; Ryu, J-C.; Kim, Y. Eur. J. Med. Chem. 2003, 38, 537.
[8] Du, Z.; Ng, H.; Zhang, K.; Zeng, H.; Wang, J. Org. Biomol. Chem. 2011, 9, 6930.
[9] Tang, E.; Chen, B.; Zhang, L.; Li, W.; Lin, J. Synlett 2011, 707.
[10] Ahmed, N.; van Lier, J. E. Tetrahedron Lett. 2007, 48, 13.
[11] Kim, D.; Ham, K.; Hong, S. Org. Biomol. Chem. 2012, 10, 7305.
[12] Wu, X.-F.; Neumann, H.; Beller, M. Chem. Eur. J. 2012, 18, 12595.
[13] Yatabe, T.; Jin, X.; Yamaguchi, K.; Mizuno, N. Angew. Chem., Int. Ed. 2015, 54, 13302.
[14] Ma, M.-L.; Li, M.; Gou, J.-J.; Ruan, T.-Y.; Jin, H.-S.; Zhang, L.-H.; Wu, L.-C.; Li, X.-Y.; Hu, Y.-H.; Zhao, Z. Bioorg. Med. Chem. 2014, 22, 6117.
[15] Huang, X.; Tang, E.; Xu, W.-M.; Cao, J. J. Comb. Chem. 2005, 7, 802.
[16] Guz, N. R.; Stermitz, F. R.; Johnson, J. B.; Beeson, T. D.; Willen, S.; Hsiang, J-F.; Lewis, K. J. Med. Chem. 2001, 44, 261.
[17] Lin, Y.; Zhou, Y.; Flavin, M. T.; Zhou, L.; Nie, W.; Chen, F. Bioorg. Med. Chem. 2002, 10, 2795.
[18] Hu, W.; Lin, J. P.; Song, L. R.; Long, Y. Q. Org. Lett. 2015, 17, 1268.
[19] An, Z.-Y.; Yan, Y.-Y.; Peng, D.; Ou, T.-M.; Huang, S.-L.; An, L.-K.; Gu, L.-Q.; Huang, Z.-S. Eur. J. Med. Chem. 2010, 45, 3895.
[20] Ding, D.; Li, X.; Wang, X.; Du, Y.; Shen, J. Tetrahedron Lett. 2006, 39, 6997.
[21] Huang, J.; Chen, Y.; King, A. O.; Dilmeghani, M.; Larsen, R. D.; Faul, M. M. Org. Lett. 2008, 10, 2609.
[22] Jones, C. P.; Anderson, K. W.; Buchwald, S. L. J. Org. Chem. 2007, 72, 7968.
[23] Sun, F.; Zhao, X.; Shi, D. Tetrahedron Lett. 2011, 52, 5633.
/
| 〈 |
|
〉 |